Intestinal myofibroblasts: targets for stem cell therapy
- PMID: 21252048
- PMCID: PMC3094146
- DOI: 10.1152/ajpgi.00474.2010
Intestinal myofibroblasts: targets for stem cell therapy
Abstract
The subepithelial intestinal myofibroblast is an important cell orchestrating many diverse functions in the intestine and is involved in growth and repair, tumorigenesis, inflammation, and fibrosis. The myofibroblast is but one of several α-smooth muscle actin-positive (α-SMA(+)) mesenchymal cells present within the intestinal lamina propria, including vascular pericytes, bone marrow-derived stem cells (mesenchymal stem cells or hematopoietic stem cells), muscularis mucosae, and the lymphatic pericytes (colon) and organized smooth muscle (small intestine) associated with the lymphatic lacteals. These other mesenchymal cells perform many of the functions previously attributed to subepithelial myofibroblasts. This review discusses the definition of a myofibroblast and reconsiders whether the α-SMA(+) subepithelial cells in the intestine are myofibroblasts or other types of mesenchymal cells, i.e., pericytes. Current information about specific, or not so specific, molecular markers of lamina propria mesenchymal cells is reviewed, as well as the origins of intestinal myofibroblasts and pericytes in the intestinal lamina propria and their replenishment after injury. Current concepts and research on stem cell therapy for intestinal inflammation are summarized. Information about the stem cell origin of intestinal stromal cells may inform future stem cell therapies to treat human inflammatory bowel disease (IBD).
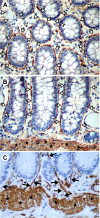
Figures




References
-
- Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001 - PubMed
-
- Adegboyega PA, Mifflin RC, DiMari JF, Saada JI, Powell DW. Immunohistochemical study of myofibroblasts in normal colonic mucosa, hyperplastic polyps, and adenomatous colorectal polyps. Arch Pathol Lab Med 126: 829–836, 2002 - PubMed
-
- Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res 10: 5870–5879, 2004 - PubMed
-
- Aertgeerts K, Levin I, Shi L, Snell GP, Jennings A, Prasad GS, Zhang Y, Kraus ML, Salakian S, Sridhar V, Wijnands R, Tennant MG. Structural and kinetic analysis of the substrate specificity of human fibroblast activation protein alpha. J Biol Chem 280: 19441–19444, 2005 - PubMed
-
- Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Therapeut 114: 94–106, 2007 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

