Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice
- PMID: 21252091
- PMCID: PMC3062310
- DOI: 10.1182/blood-2010-08-301507
Development of human CD4+FoxP3+ regulatory T cells in human stem cell factor-, granulocyte-macrophage colony-stimulating factor-, and interleukin-3-expressing NOD-SCID IL2Rγ(null) humanized mice
Abstract
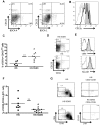
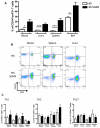
Human hematolymphoid mice have become valuable tools for the study of human hematopoiesis and uniquely human pathogens in vivo. Recent improvements in xenorecipient strains allow for long-term reconstitution with a human immune system. However, certain hematopoietic lineages, for example, the myeloid lineage, are underrepresented, possibly because of the limited cross-reactivity of murine and human cytokines. Therefore, we created a nonobese diabetic/severe combined immunodeficiency/interleukin-2 receptor-γ-null (NOD-SCID IL2Rγ(null)) mouse strain that expressed human stem cell factor, granulocyte-macrophage colony-stimulating factor, and interleukin-3, termed NSG-SGM3. Transplantation of CD34(+) human hematopoietic stem cells into NSG-SGM3 mice led to robust human hematopoietic reconstitution in blood, spleen, bone marrow, and liver. Human myeloid cell frequencies, specifically, myeloid dendritic cells, were elevated in the bone marrow of humanized NSG-SGM3 mice compared with nontransgenic NSG recipients. Most significant, however, was the increase in the CD4(+)FoxP3(+) regulatory T-cell population in all compartments analyzed. These CD4(+)FoxP3(+) regulatory T cells were functional, as evidenced by their ability to suppress T-cell proliferation. In conclusion, humanized NSG-SGM3 mice might serve as a useful model to study human regulatory T-cell development in vivo, but this unexpected lineage skewing also highlights the importance of adequate spatiotemporal expression of human cytokines for future xenorecipient strain development.
Figures
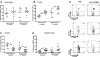




References
-
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7(2):118–130. - PubMed
-
- Traggiai E, Chicha L, Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304(5667):104–107. - PubMed
-
- Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–6489. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

