Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload
- PMID: 21252154
- PMCID: PMC3065330
- DOI: 10.1161/CIRCRESAHA.110.221911
Reactive oxygen species-activated Ca/calmodulin kinase IIδ is required for late I(Na) augmentation leading to cellular Na and Ca overload
Abstract
Rationale: In heart failure Ca/calmodulin kinase (CaMK)II expression and reactive oxygen species (ROS) are increased. Both ROS and CaMKII can increase late I(Na) leading to intracellular Na accumulation and arrhythmias. It has been shown that ROS can activate CaMKII via oxidation.
Objective: We tested whether CaMKIIδ is required for ROS-dependent late I(Na) regulation and whether ROS-induced Ca released from the sarcoplasmic reticulum (SR) is involved.
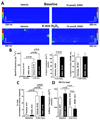
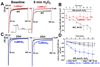
Methods and results: 40 μmol/L H(2)O(2) significantly increased CaMKII oxidation and autophosphorylation in permeabilized rabbit cardiomyocytes. Without free [Ca](i) (5 mmol/L BAPTA/1 mmol/L Br(2)-BAPTA) or after SR depletion (caffeine 10 mmol/L, thapsigargin 5 μmol/L), the H(2)O(2)-dependent CaMKII oxidation and autophosphorylation was abolished. H(2)O(2) significantly increased SR Ca spark frequency (confocal microscopy) but reduced SR Ca load. In wild-type (WT) mouse myocytes, H(2)O(2) increased late I(Na) (whole cell patch-clamp). This increase was abolished in CaMKIIδ(-/-) myocytes. H(2)O(2)-induced [Na](i) and [Ca](i) accumulation (SBFI [sodium-binding benzofuran isophthalate] and Indo-1 epifluorescence) was significantly slowed in CaMKIIδ(-/-) myocytes (versus WT). CaMKIIδ(-/-) myocytes developed significantly less H(2)O(2)-induced arrhythmias and were more resistant to hypercontracture. Opposite results (increased late I(Na), [Na](i) and [Ca](i) accumulation) were obtained by overexpression of CaMKIIδ in rabbit myocytes (adenoviral gene transfer) reversible with CaMKII inhibition (10 μmol/L KN93 or 0.1 μmol/L AIP [autocamtide 2-related inhibitory peptide]).
Conclusions: Free [Ca](i) and a functional SR are required for ROS activation of CaMKII. ROS-activated CaMKIIδ enhances late I(Na), which may lead to cellular Na and Ca overload. This may be of relevance in hear failure, where enhanced ROS production meets increased CaMKII expression.
Figures





References
-
- Tsutsui H, Ide T, Hayashidani S, Suematsu N, Utsumi H, Nakamura R, Egashira K, Takeshita A. Greater susceptibility of failing cardiac myocytes to oxygen free radical-mediated injury. Cardiovasc Res. 2001;49:103–109. - PubMed
-
- Kinugawa S, Tsutsui H, Hayashidani S, Ide T, Suematsu N, Satoh S, Utsumi H, Takeshita A. Treatment with dimethylthiourea prevents left ventricular remodeling and failure after experimental myocardial infarction in mice: Role of oxidative stress. Circ Res. 2000;87:392–398. - PubMed
-
- Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A. Elevated levels of 8-iso-prostaglandin f-2 alpha in pericardial fluid of patients with heart failure - a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation. 1998;97:1536–1539. - PubMed
-
- Josephson R, Silverman H, Lakatta E, Stern M, Zweier J. Study of the mechanisms of hydrogen-peroxide and hydroxyl free radical-induced cellular injury and calcium overload in cardiac myocytes. J Biol Chem. 1991;266:2354–2361. - PubMed
-
- Wagner S, Seidler T, Picht E, Maier LS, Kazanski V, Teucher N, Schillinger W, Pieske B, Isenberg G, Hasenfuss G, Kögler H. Na+- Ca2+ exchanger overexpression predisposes to reactive oxygen species-induced injury. Cardiovasc Res. 2003;60:404–412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

