Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes
- PMID: 21252909
- PMCID: PMC3131963
- DOI: 10.1038/cdd.2010.184
Diacylglycerol kinase α regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes
Abstract
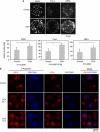
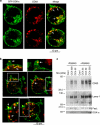
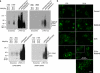
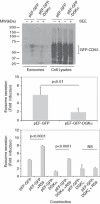
Multivesicular bodies (MVBs) are endocytic compartments that contain intraluminal vesicles formed by inward budding from the limiting membrane of endosomes. In T lymphocytes, these vesicles contain pro-apoptotic Fas ligand (FasL), which may be secreted as 'lethal exosomes' upon fusion of MVBs with the plasma membrane. Diacylglycerol kinase α (DGKα) regulate the secretion of exosomes, but it is unclear how this control is mediated. T-lymphocyte activation increases the number of MVBs that contain FasL. DGKα is recruited to MVBs and to exosomes in which it has a double function. DGKα kinase activity exerts a negative role in the formation of mature MVBs, as we demonstrate by the use of an inhibitor. Downmodulation of DGKα protein resulted in inhibition of both the polarisation of MVBs towards immune synapse and exosome secretion. The subcellular location of DGKα together with its complex role in the formation and polarised traffic of MVBs support the notion that DGKα is a key regulator of the polarised secretion of exosomes.
Figures



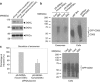
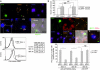
References
-
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. - PubMed
-
- Bossi G, Griffiths GM. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat Med. 1999;5:90–96. - PubMed
-
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. - PubMed
-
- Zuccato E, Blott EJ, Holt O, Sigismund S, Shaw M, Bossi G, et al. Sorting of Fas ligand to secretory lysosomes is regulated by mono-ubiquitylation and phosphorylation. J Cell Sci. 2007;120:191–199. - PubMed
-
- Monleon I, Martinez-Lorenzo MJ, Monteagudo L, Lasierra P, Taules M, Iturralde M, et al. Differential secretion of Fas ligand- or APO2 ligand/TNF-related apoptosis-inducing ligand-carrying microvesicles during activation-induced death of human T cells. J Immunol. 2001;167:6736–6744. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

