Autocrine motility factor/phosphoglucose isomerase regulates ER stress and cell death through control of ER calcium release
- PMID: 21252914
- PMCID: PMC3131941
- DOI: 10.1038/cdd.2010.181
Autocrine motility factor/phosphoglucose isomerase regulates ER stress and cell death through control of ER calcium release
Abstract
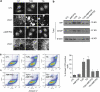
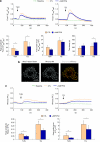
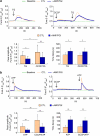
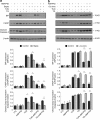
Autocrine motility factor/ phosphoglucose isomerase (AMF/PGI) promotes cell survival by the pAkt survival pathway. Its receptor, gp78/AMFR, is an E3 ubiquitin ligase implicated in endoplasmic reticulum (ER)-associated protein degradation. We demonstrate here that AMF/PGI also protects against thapsigargin (TG)- and tunicamycin (TUN)-induced ER stress and apoptosis. AMF/PGI protection against the ER stress response is receptor mediated as it is not observed in gp78/AMFR-knockdown HEK293 cells. However, AMF/PGI protection against the ER stress response by TG and TUN was mediated only partially through PI3K/Akt activation. AMF/PGI reduction of the elevation of cytosolic calcium in response to either TG or inositol 1,4,5-trisphosphate receptor activation with ATP was gp78/AMFR-dependent, independent of mitochondrial depolarization and not associated with changes in ER calcium content. These results implicate regulation of ER calcium release in AMF/PGI protection against ER stress and apoptosis. Indeed, sequestration of cytosolic calcium with BAPTA-AM limited the ER stress response. Importantly, elevation of cytosolic calcium upon treatment with the calcium ionophore ionomycin, while not inducing an ER stress response, did prevent AMF/PGI protection against ER stress. By regulating ER calcium release, AMF/PGI interaction with gp78/AMFR therefore protects against ER stress identifying novel roles for these cancer-associated proteins in promoting tumor cell survival.
Figures






References
-
- Ellgaard L, Helenius A. Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol. 2003;4:181–191. - PubMed
-
- Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ. 2006;13:363–373. - PubMed
-
- Bernales SN, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

