Hepatocellular carcinoma xenograft supports HCV replication: a mouse model for evaluating antivirals
- PMID: 21253388
- PMCID: PMC3022289
- DOI: 10.3748/wjg.v17.i3.300
Hepatocellular carcinoma xenograft supports HCV replication: a mouse model for evaluating antivirals
Erratum in
-
Correction to: Hepatocellular carcinoma xenograft supports HCV replication: A mouse model for evaluating antivirals.World J Gastroenterol. 2025 Jun 21;31(23):108472. doi: 10.3748/wjg.v31.i23.108472. World J Gastroenterol. 2025. PMID: 40575336 Free PMC article.
Abstract
Aim: To develop a hepatocellular carcinoma (HCC) xenograft model for studying hepatitis C virus (HCV) replication in a mice, and antiviral treatment.
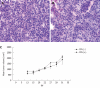
Methods: We developed a stable S3-green fluorescence protein (GFP) cell line that replicated the GFP-tagged HCV sub-genomic RNA derived from a highly efficient JFH1 virus. S3-GFP replicon cell line was injected subcutaneously into γ-irradiated SCID mice. We showed that the S3-GFP replicon cell line formed human HCC xenografts in SCID mice. Cells were isolated from subcutaneous tumors and then serially passaged multiple times in SCID mice by culturing in growth medium supplemented with G-418. The mouse-adapted S3-GFP replicon cells were implanted subcutaneously and also into the liver of SCID mice via intrasplenic infusion to study the replication of HCV in the HCC xenografts. The tumor model was validated for antiviral testing after intraperitoneal injection of interferon-α (IFN-α).
Results: A highly tumorigenic S3-GFP replicon cell line was developed that formed subcutaneous tumors within 2 wk and diffuse liver metastasis within 4 wk in SCID mice. Replication of HCV in the subcutaneous and liver tumors was confirmed by cell colony assay, detection of the viral RNA by ribonuclease protection assay and real-time quantitative reverse transcription polymerase chain reaction. High-level replication of HCV sub-genomic RNA in the tumor could be visualized by GFP expression using fluorescence microscopy. IFN-α cleared HCV RNA replication in the subcutaneous tumors within 2 wk and 4 wk in the liver tumor model.
Conclusion: A non-infectious mouse model allows us to study replication of HCV in subcutaneous and metastatic liver tumors. Clearance of HCV by IFN-α supports use of this model to test other anti-HCV drugs.
Keywords: Antiviral agent; Hepatitis C virus; Hepatocellular carcinoma; Interferon-α; SCID mouse; Tumor xenograft; Virus replication.
Figures





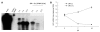


References
-
- Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. - PubMed
-
- Poynard T, Yuen MF, Ratziu V, Lai CL. Viral hepatitis C. Lancet. 2003;362:2095–2100. - PubMed
-
- Webster DP, Klenerman P, Collier J, Jeffery KJ. Development of novel treatments for hepatitis C. Lancet Infect Dis. 2009;9:108–117. - PubMed
-
- Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979–1998. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

