Bones and Crohn's: no benefit of adding sodium fluoride or ibandronate to calcium and vitamin D
- PMID: 21253392
- PMCID: PMC3022293
- DOI: 10.3748/wjg.v17.i3.334
Bones and Crohn's: no benefit of adding sodium fluoride or ibandronate to calcium and vitamin D
Abstract
Aim: To compare the effect of calcium and cholecalciferol alone and along with additional sodium fluoride or ibandronate on bone mineral density (BMD) and fractures in patients with Crohn's disease (CD).
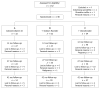
Methods: Patients (n =148) with reduced BMD (T-score < -1) were randomized to receive cholecalciferol (1000 IU) and calcium citrate (800 mg) daily alone(group A, n = 32) or along with additional sodium fluoride (25 mg bid) (group B, n = 62) or additional ibandronate (1 mg iv/3-monthly) (group C, n = 54). Dual energy X-ray absorptiometry of the lumbar spine (L1-L4) and proximal right femur and X-rays of the spine were performed at baseline and after 1.0, 2.25 and 3.5 years. Fracture-assessment included visual reading of X-rays and quantitative morphometry of vertebral bodies (T4-L4).
Results: One hundred and twenty three (83.1%) patients completed the first year for intention-to-treat (ITT) analysis. Ninety two (62.2%) patients completed the second year and 71 (47.8%) the third year available for per-protocol (PP) analysis. With a significant increase in T-score of the lumbar spine by +0.28 ± 0.35 [95% confidence interval (CI): 0.162-0.460, P < 0.01], +0.33 ± 0.49 (95% CI: 0.109-0.558, P < 0.01), +0.43 ± 0.47 (95% CI: 0.147-0.708, P < 0.01) in group A, +0.22 ± 0.33 (95% CI: 0.125-0.321, P < 0.01); +0.47 ± 0.60 (95% CI: 0.262-0.676, P < 0.01), +0.51 ± 0.44 (95% CI: 0.338-0.682, P < 0.01) in group B and +0.22 ± 0.38 (95% CI: 0.111-0.329, P < 0.01), +0.36 ± 0.53 (95% CI: 0.147-0.578, P < 0.01), +0.41 ± 0.48 (95% CI: 0.238-0.576, P < 0.01) in group C, respectively, during the 1.0, 2.25 and 3.5 year periods (PP analysis), no treatment regimen was superior in any in- or between-group analyses. In the ITT analysis, similar results in all in- and between-group analyses with a significant in-group but non-significant between-group increase in T-score of the lumbar spine by 0.38 ± 0.46 (group A, P < 0.01), 0.37 ± 0.50 (group B, P < 0.01) and 0.35 ± 0.49 (group C, P < 0.01) was observed. Follow-up in ITT analysis was still 2.65 years. One vertebral fracture in the sodium fluoride group was detected. Study medication was safe and well tolerated.
Conclusion: Additional sodium fluoride or ibandronate had no benefit over calcium and cholecalciferol alone in managing reduced BMD in CD.
Keywords: Bone mineral density; Calcium; Cholecalciferol; Crohn’s disease; Ibandronate; Sodium fluoride; Vertebral fracture.
Figures


References
-
- Abitbol V, Roux C, Chaussade S, Guillemant S, Kolta S, Dougados M, Couturier D, Amor B. Metabolic bone assessment in patients with inflammatory bowel disease. Gastroenterology. 1995;108:417–422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

