The Saccharomyces cerevisiae Genes, AIM45, YGR207c/CIR1 and YOR356w/CIR2, Are Involved in Cellular Redox State Under Stress Conditions
- PMID: 21253464
- PMCID: PMC3023949
- DOI: 10.2174/1874285801004010075
The Saccharomyces cerevisiae Genes, AIM45, YGR207c/CIR1 and YOR356w/CIR2, Are Involved in Cellular Redox State Under Stress Conditions
Abstract
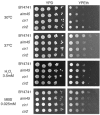
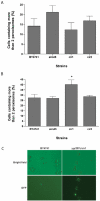
Mammalian electron transfer flavoproteins comprise a mitochondrial matrix heterodimer, and an electron transfer flavoprotein dehydrogenase localized in the mitochondrial inner membrane. Electrons from primary acyl-CoA dehydrogenases, of mitochondrial metabolism of fatty acids and amino acids, are transferred to the matricial heterodimer and, subsequently, to the electron transfer flavoprotein dehydrogenase, which transfers electrons to ubiquinone of the mitochondrial electron transport chain. Several evidences suggest that these proteins may convey electrons directly to molecular oxygen, yielding reactive oxygen species. In this work, we investigated phenotypes of the yeast mutants affected in the orthologous genes of the matrix heterodimer (AIM45 and YGR207c/CIR1) and of the electron transfer flavoprotein dehydrogenase (YOR356w/CIR2). The mutant strains aim45 and yor356w/cir2 displayed better growth on several non-fermentable carbon sources, which depended on the component of the electron transport chain that accepts the electrons resulting from its mitochondrial oxidation. Furthermore, upon heat shock, the mutant strains presented decreased intracellular oxidation, suggesting that these flavoproteins are a source of reactive oxygen species. Other phenotypes identified suggest that AIM45, YGR207c/CIR1 and YOR356w/CIR2 can protect cells from oxidative and heat stress, which encompass increased heat stress sensitivity, superoxide sensitivity, both only on non-fermentable carbon sources.
Keywords: Electron transfer flavoproteins (ETF); intracellular oxidation under stress conditions.; mitochondrion.
Figures


References
-
- Reinders J, Zahedi RP, Pfanner N, Meisinger C, Sickmann A. Toward the complete yeast mitochondrial proteome: multidimensional separation techniques for mitochondrial proteomics. J Proteome Res. 2006;5:1543–54. - PubMed
-
- Toogood HS, Leys D, Scrutton NS. Dynamics driving function: new insights from electron transferring flavoproteins and partner complexes. FEBS J. 2007;274:5481–504. - PubMed
-
- Mattoon JR, Haight RD. Glutaric acid accumulation by a lysine-requiring yeast mutant. J Biol Chem. 1962;237:3486–90. - PubMed
-
- Spaan AN, Ijlst L, van Roermund CWT, Wijburg FA, Wanders RJA, Waterham HR. Identification of the human mitochondrial FAD transporter and its potential role in multiple acyl-CoA dehydrogenase deficiency. Mol Genet Metab. 2005;86:441–7. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
