Effects of IFN-B on TRAIL and Decoy Receptor Expression in Different Immune Cell Populations from MS Patients with Distinct Disease Subtypes
- PMID: 21253524
- PMCID: PMC3022173
- DOI: 10.4061/2011/485752
Effects of IFN-B on TRAIL and Decoy Receptor Expression in Different Immune Cell Populations from MS Patients with Distinct Disease Subtypes
Abstract
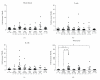
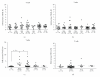
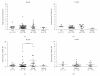
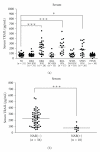
Using quantitative RT-PCR, we compared mRNA levels for TRAIL [tumor necrosis factor (TNF)-related apoptosis-inducing ligand] and its receptors in various immune cell subsets derived from the peripheral blood of untreated normal subjects (NS) and patients with distinct subtypes of multiple sclerosis (MS): active relapsing-remitting MS (RRA), quiescent relapsing-remitting MS (RRQ), secondary-progressive MS (SPMS) or primary-progressive MS (PPMS). Consistent with a role for TRAIL in the mechanism of action of interferon-β (IFN-β), TRAIL mRNA levels were increased in monocytes from patients clinically responsive to IFN-β (RRQ) but not those unresponsive to this therapeutic (RRA). TRAIL-R3 (decoy receptor) expression was elevated in T cells from untreated RRMS patients while IFN-β therapy reversed this increase suggesting that IFN-β may promote the apoptotic elimination of autoreactive T cells by increasing the amount of TRAIL available to activate TRAIL death receptors. Serum concentrations of soluble TRAIL were increased to a similar extent by IFN-β therapy in RRQ, RRA and SPMS patients that had not generated neutralizing antibodies against this cytokine. Although our findings suggest altered TRAIL signaling may play a role in MS pathogenesis and IFN-β therapy, they do not support use of TRAIL as a surrogate marker for clinical responsiveness to this therapeutic.
Figures
Similar articles
-
Candidate gene study of TRAIL and TRAIL receptors: association with response to interferon beta therapy in multiple sclerosis patients.PLoS One. 2013 Apr 29;8(4):e62540. doi: 10.1371/journal.pone.0062540. Print 2013. PLoS One. 2013. PMID: 23658636 Free PMC article.
-
The role of cell type-specific responses in IFN-β therapy of multiple sclerosis.Proc Natl Acad Sci U S A. 2011 Dec 6;108(49):19689-94. doi: 10.1073/pnas.1117347108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106296 Free PMC article.
-
Distinct chemokine receptor and cytokine expression profile in secondary progressive MS.Neurology. 2001 Oct 23;57(8):1371-6. doi: 10.1212/wnl.57.8.1371. Neurology. 2001. PMID: 11673573
-
Apoptosis mediators fasL and TRAIL are upregulated in peripheral blood mononuclear cells in MS.Neurology. 2000 Oct 10;55(7):928-34. doi: 10.1212/wnl.55.7.928. Neurology. 2000. PMID: 11061246
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
Cited by
-
The Proteasome Inhibitor Marizomib Evokes Endoplasmic Reticulum Stress and Promotes Apoptosis in Human Glioblastoma Cells.Pharmaceuticals (Basel). 2024 Aug 20;17(8):1089. doi: 10.3390/ph17081089. Pharmaceuticals (Basel). 2024. PMID: 39204194 Free PMC article.
-
The role of TRAIL in fatigue induced by repeated stress from radiotherapy.J Psychiatr Res. 2017 Aug;91:130-138. doi: 10.1016/j.jpsychires.2017.03.012. Epub 2017 Mar 20. J Psychiatr Res. 2017. PMID: 28343068 Free PMC article.
-
Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line.Tumour Biol. 2016 Jan;37(1):931-42. doi: 10.1007/s13277-015-3781-8. Epub 2015 Aug 11. Tumour Biol. 2016. PMID: 26260271 Free PMC article.
-
TRAIL and TRAIL receptors splice variants during long-term interferon β treatment of patients with multiple sclerosis: evaluation as biomarkers for therapeutic response.J Neurol Neurosurg Psychiatry. 2016 Feb;87(2):130-7. doi: 10.1136/jnnp-2014-309932. Epub 2015 Mar 3. J Neurol Neurosurg Psychiatry. 2016. PMID: 25736057 Free PMC article.
-
Candidate gene study of TRAIL and TRAIL receptors: association with response to interferon beta therapy in multiple sclerosis patients.PLoS One. 2013 Apr 29;8(4):e62540. doi: 10.1371/journal.pone.0062540. Print 2013. PLoS One. 2013. PMID: 23658636 Free PMC article.
References
-
- Neumann H. Molecular mechanisms of axonal damage in inflammatory central nervous system diseases. Current Opinion in Neurology. 2003;16(3):267–273. - PubMed
-
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. The New England Journal of Medicine. 2000;343(13):938–952. - PubMed
-
- Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219(4582):308–310. - PubMed
-
- Vizler C, Bercovici N, Cornet A, Cambouris C, Liblau RS. Role of autoreactive CD8+ T cells in organ-specific autoimmune diseases: insight from transgenic mouse models. Immunological Reviews. 1999;169:81–92. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

