Biofilm development on Caenorhabditis elegans by Yersinia is facilitated by quorum sensing-dependent repression of type III secretion
- PMID: 21253572
- PMCID: PMC3017118
- DOI: 10.1371/journal.ppat.1001250
Biofilm development on Caenorhabditis elegans by Yersinia is facilitated by quorum sensing-dependent repression of type III secretion
Abstract
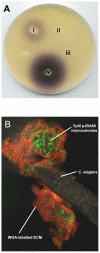
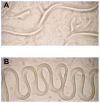
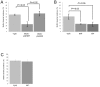
Yersinia pseudotuberculosis forms biofilms on Caenorhabditis elegans which block nematode feeding. This genetically amenable host-pathogen model has important implications for biofilm development on living, motile surfaces. Here we show that Y. pseudotuberculosis biofilm development on C. elegans is governed by N-acylhomoserine lactone (AHL)-mediated quorum sensing (QS) since (i) AHLs are produced in nematode associated biofilms and (ii) Y. pseudotuberculosis strains expressing an AHL-degrading enzyme or in which the AHL synthase (ypsI and ytbI) or response regulator (ypsR and ytbR) genes have been mutated, are attenuated. Although biofilm formation is also attenuated in Y. pseudotuberculosis strains carrying mutations in the QS-controlled motility regulator genes, flhDC and fliA, and the flagellin export gene, flhA, flagella are not required since fliC mutants form normal biofilms. However, in contrast to the parent and fliC mutant, Yop virulon proteins are up-regulated in flhDC, fliA and flhA mutants in a temperature and calcium independent manner. Similar observations were found for the Y. pseudotuberculosis QS mutants, indicating that the Yop virulon is repressed by QS via the master motility regulator, flhDC. By curing the pYV virulence plasmid from the ypsI/ytbI mutant, by growing YpIII under conditions permissive for type III needle formation but not Yop secretion and by mutating the type III secretion apparatus gene, yscJ, we show that biofilm formation can be restored in flhDC and ypsI/ytbI mutants. These data demonstrate that type III secretion blocks biofilm formation and is reciprocally regulated with motility via QS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



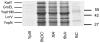

References
-
- Navarro L, Alto NM, Dixon JE. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr Opin Microbiol. 2005;8:21–27. - PubMed
-
- Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. - PubMed
-
- Ramamurthi KS, Schneewind O. Type III protein secretion in Yersinia species. Annu Rev Cell Dev Biol. 2002;18:107–133. - PubMed
-
- Darby C, Hsu JW, Ghori N, Falkow S. Caenorhabditis elegans - Plague bacteria biofilm blocks food intake. Nature. 2002;417:243–244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

