Increasing off-label use of antipsychotic medications in the United States, 1995-2008
- PMID: 21254289
- PMCID: PMC3069498
- DOI: 10.1002/pds.2082
Increasing off-label use of antipsychotic medications in the United States, 1995-2008
Abstract
Objective: To evaluate patterns of antipsychotic use. DESIGN, SETTING, AND MEASUREMENTS: We used nationally representative data from the IMS Health National Disease and Therapeutic Index to describe outpatient antipsychotic use. The primary outcome was the volume of visits where antipsychotics were used for specific indications (treatment visits). We also quantified use without U.S. Food and Drug Administration approval (off-label use) and off-label use with compendium data suggesting an uncertain evidence base.
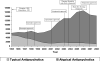
Results: Antipsychotic use increased from 6.2 million (M) treatment visits (95% CI, 5.4-7.0) in 1995 to 16.7 M visits (15.5-18.2) in 2006, then declined to 14.3 M visits (13.0-15.6) by 2008. A shift occurred from typical agents in 1995 (84% of all antipsychotic visits) to atypical agents by 2008 (93%). As they declined, typical medications shifted toward use in schizophrenia (30% in 1995 to 48% 2008). In contrast, use of atypical agents expanded for bipolar affective disorder (10 to 34%), remained stable for depression (12 to 14%), and declined for schizophrenia (56 to 23%). Overall, antipsychotic use for indications without FDA approval increased from 4.4 M visits in 1995 to 9.0 M in 2008. The estimated cost associated with off-label use in 2008 was US$6.0 billion.
Conclusions: Atypical use has grown far beyond substitution for the now infrequently used typical agents. Antipsychotics are increasingly used for conditions where FDA approval and associated clinical evidence is less certain. Despite the value of innovation, the benefits of widening atypical antipsychotic use should be weighed against their cost, regulatory status, and incomplete nature of available evidence.
Copyright © 2011 John Wiley & Sons, Ltd.
Figures
References
-
- Prescription Drug Trends The Kaiser Family Foundation, 2007. 2007. [February 3, 2009]. Available at: http://www.kff.org/rxdrugs/upload/3057_06.pdf.
-
- McDonagh MS, Peterson K, Carson S, Chan B, Thakurta S. Drug class review on atypical antipsychotic drugs. Update #2 final report. [March 24, 2010]. http://www.ohsu.edu/drugeffectiveness/reports/final.cfm. - PubMed
-
- Briesacher BA, Limcangco MR, Simoni-Wastila L, et al. The quality of antipsychotic prescribing in nursing homes. Archives of Internal Medicine. 2005;165:1280–1285. - PubMed
-
- Zuvekas SH. Prescription drugs and the changing patterns of treatment for mental disorders, 1996-2001. Health Affairs. 2005;24:195–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials