The cell cycle of Leishmania: morphogenetic events and their implications for parasite biology
- PMID: 21255109
- PMCID: PMC3166656
- DOI: 10.1111/j.1365-2958.2010.07479.x
The cell cycle of Leishmania: morphogenetic events and their implications for parasite biology
Abstract
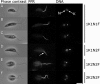
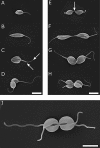
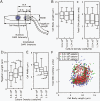
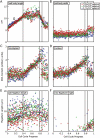
The cell cycle is central to understanding fundamental biology of Leishmania, a group of human-infective protozoan parasites. Leishmania have two main life cycle morphologies: the intracellular amastigote in the mammalian host and the promastigote in the fly. We have produced the first comprehensive and quantitative description of a Leishmania promastigote cell cycle taking a morphometric approach to position any cell within the cell cycle based on its length and DNA content. We describe timings of cell cycle phases and rates of morphological changes; kinetoplast and nucleus S phase, division and position, cell body growth and morphology changes, flagellum growth and basal body duplication. We have shown that Leishmania mexicana undergoes large changes in morphology through the cell cycle and that the wide range of morphologies present in cultures during exponential growth represent different cell cycle stages. We also show promastigote flagellum growth occurs over multiple cell cycles. There are clear implications for the mechanisms of flagellum length regulation, life cycle stage differentiation and trypanosomatid division in general. This data set therefore provides a platform which will be of use for post-genomic analyses of Leishmania cell biology in relation to differentiation and infection.
© 2010 Blackwell Publishing Ltd.
Figures


References
-
- Antoine J, Prina E, Courret N, Lang T. Leishmania spp.: on the interactions they establish with antigen-presenting cells of their mammalian hosts. Adv Parasitol. 2004;58:1–68. - PubMed
-
- Bastin P, Ellis K, Kohl L, Gull K. Flagellum ontogeny in trypanosomes studied via an inherited and regulated RNA interference system. J Cell Sci. 2000;113(Part 18):3321–3328. - PubMed
-
- Bates PA. Complete developmental cycle of Leishmania mexicana in axenic culture. Parasitology. 1994;108:1–9. - PubMed
-
- Bengs F, Scholz A, Kuhn D, Wiese M. LmxMPK9, a mitogen-activated protein kinase homologue affects flagellar length in Leishmania mexicana. Mol Microbiol. 2005;55:1606–1615. - PubMed
-
- Berriman M, Ghedin E, Hertz-Fowler C, Blandin G, Renauld H, Bartholomeu DC, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

