Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis
- PMID: 21255120
- PMCID: PMC3312031
- DOI: 10.1111/j.1365-2958.2010.07490.x
Characterization of a new cytotoxin that contributes to Staphylococcus aureus pathogenesis
Abstract
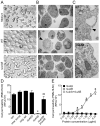
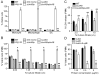
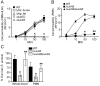
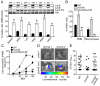
Staphylococcus aureus is an important pathogen that continues to be a significant global health threat because of the prevalence of methicillin-resistant S. aureus strains (MRSA). The pathogenesis of this organism is partly attributed to the production of a large repertoire of cytotoxins that target and kill innate immune cells, which provide the first line of defence against S. aureus infection. Here we demonstrate that leukocidin A/B (LukAB) is required and sufficient for the ability of S. aureus, including MRSA, to kill human neutrophils, macrophages and dendritic cells. LukAB targets the plasma membrane of host cells resulting in cellular swelling and subsequent cell death. We found that S. aureus lacking lukAB are severely impaired in their ability to kill phagocytes during bacteria-phagocyte interaction, which in turn renders the lukAB-negative staphylococci more susceptible to killing by neutrophils. Notably, we show that lukAB is expressed in vivo within abscesses in a murine infection model and that it contributes significantly to pathogenesis of MRSA in an animal host. Collectively, these results extend our understanding of how S. aureus avoids phagocyte-mediated clearance, and underscore LukAB as an important factor that contributes to staphylococcal pathogenesis.
© 2010 Blackwell Publishing Ltd.
Figures

References
-
- Baba T, Takeuchi F, Kuroda M, Yuzawa H, Aoki K, Oguchi A, Nagai Y, Iwama N, Asano K, Naimi T, Kuroda H, Cui L, Yamamoto K, Hiramatsu K. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. - PubMed
-
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. - PubMed
-
- Brown EL, Dumitrescu O, Thomas D, Badiou C, Koers EM, Choudhury P, Vazquez V, Etienne J, Lina G, Vandenesch F, Bowden MG. The Panton-Valentine leukocidin vaccine protects mice against lung and skin infections caused by Staphylococcus aureus USA300. Clin Microbiol Infect. 2009;15:156–164. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

