A nuclear export signal within the structural Gag protein is required for prototype foamy virus replication
- PMID: 21255441
- PMCID: PMC3033328
- DOI: 10.1186/1742-4690-8-6
A nuclear export signal within the structural Gag protein is required for prototype foamy virus replication
Abstract
Background: The Gag polyproteins play distinct roles during the replication cycle of retroviruses, hijacking many cellular machineries to fulfill them. In the case of the prototype foamy virus (PFV), Gag structural proteins undergo transient nuclear trafficking after their synthesis, returning back to the cytoplasm for capsid assembly and virus egress. The functional role of this nuclear stage as well as the molecular mechanism(s) responsible for Gag nuclear export are not understood.
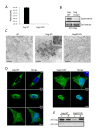
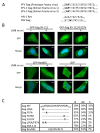
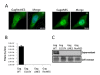
Results: We have identified a leptomycin B (LMB)-sensitive nuclear export sequence (NES) within the N-terminus of PFV Gag that is absolutely required for the completion of late stages of virus replication. Point mutations of conserved residues within this motif lead to nuclear redistribution of Gag, preventing subsequent virus egress. We have shown that a NES-defective PFV Gag acts as a dominant negative mutant by sequestrating its wild-type counterpart in the nucleus. Trans-complementation experiments with the heterologous NES of HIV-1 Rev allow the cytoplasmic redistribution of FV Gag, but fail to restore infectivity.
Conclusions: PFV Gag-Gag interactions are finely tuned in the cytoplasm to regulate their functions, capsid assembly, and virus release. In the nucleus, we have shown Gag-Gag interactions which could be involved in the nuclear export of Gag and viral RNA. We propose that nuclear export of unspliced and partially spliced PFV RNAs relies on two complementary mechanisms, which take place successively during the replication cycle.
Figures


References
-
- Klein KC, Reed JC, Lingappa JR. Intracellular destinies: degradation, targeting, assembly, and endocytosis of HIV Gag. AIDS Rev. 2007;9:150–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

