FKBP12 binds to acylated H-ras and promotes depalmitoylation
- PMID: 21255728
- PMCID: PMC3085165
- DOI: 10.1016/j.molcel.2011.01.001
FKBP12 binds to acylated H-ras and promotes depalmitoylation
Abstract
A cycle of palmitoylation/depalmitoylation of H-Ras mediates bidirectional trafficking between the Golgi apparatus and the plasma membrane, but nothing is known about how this cycle is regulated. We show that the prolyl isomerase (PI) FKBP12 binds to H-Ras in a palmitoylation-dependent fashion and promotes depalmitoylation. A variety of inhibitors of the PI activity of FKBP12, including FK506, rapamycin, and cycloheximide, increase steady-state palmitoylation. FK506 inhibits retrograde trafficking of H-Ras from the plasma membrane to the Golgi in a proline 179-dependent fashion, augments early GTP loading of Ras in response to growth factors, and promotes H-Ras-dependent neurite outgrowth from PC12 cells. These data demonstrate that FKBP12 regulates H-Ras trafficking by promoting depalmitoylation through cis-trans isomerization of a peptidyl-prolyl bond in proximity to the palmitoylated cysteines.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
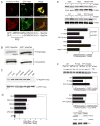


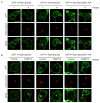
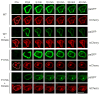
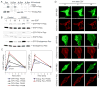
Comment in
-
Regulator of Ras depalmitoylation and retrograde trafficking: a new hat for FKBP.Mol Cell. 2011 Jan 21;41(2):131-3. doi: 10.1016/j.molcel.2011.01.009. Mol Cell. 2011. PMID: 21255722
References
-
- Baker TL, Zheng H, Walker J, Coloff JL, Buss JE. Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic H-ras. J Biol Chem. 2003;278:19292–19300. - PubMed
-
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

