Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults
- PMID: 21256197
- PMCID: PMC3052737
- DOI: 10.1016/j.neuroscience.2011.01.024
Endocannabinoid 2-arachidonoylglycerol protects neurons against β-amyloid insults
Abstract
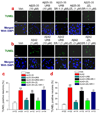
While endocannabinoid modulation of both GABAergic and glutamatergic synaptic transmission and plasticity has been extensively investigated, our understanding of the role of endocannabinoids in protecting neurons from harmful insults remains limited. 2-Arachidonoylglycerol (2-AG), the most abundant endogenous ligand and a full agonist for cannabinoid receptors, exhibits anti-inflammatory and neuroprotective effects via a CB1 receptor (CB1R)-mediated mechanism. However, it is still not clear whether 2-AG is also able to protect neurons from β-amyloid (Aβ)-induced neurodegeneration. Here, we demonstrate that exogenous application of 2-AG significantly protected hippocampal neurons in culture against Aβ-induced neurodegeneration and apoptosis. This neuroprotective effect was blocked by SR141716 (SR-1), a selective CB1R antagonist, but not by SR144528 (SR-2), a selective CB2R antagonist, or capsazepine (CAP), a selective transient receptor potential cation channels, subfamily V, member 1 (TRPV1) receptor antagonist. To determine whether endogenous 2-AG is capable of protecting neurons from Aβ insults, hippocampal neurons in culture were treated with URB602 or JZL184, selective inhibitors of monoacylglycerol lipase (MAGL), the enzyme hydrolyzing 2-AG. MAGL inhibition that elevates endogenous levels of 2-AG also significantly reduced Aβ-induced neurodegeneration and apoptosis. The 2-AG-produced neuroprotective effects appear to be mediated via CB1R-dependent suppression of extracellular signal-regulated kinases 1 and 2 (ERK1/2) and nuclear factor-κB (NF-κB) phosphorylation and cyclooxygenase-2 (COX-2) expression. Our results suggest that elevation of endogenous 2-AG by inhibiting its hydrolysis has potential as a novel efficacious therapeutic approach for preventing, ameliorating or treating Alzheimer's disease.
Copyright © 2011 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures






References
-
- Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. - PubMed
-
- Bisogno T, Di Marzo V. The role of the endocannabinoid system in Alzheimer's disease: facts and hypotheses. Curr Pharm Des. 2008;14:2299–3305. - PubMed
-
- Cabral GA, Marciano-Cabral F. Cannabinoid receptors in microglia of the central nervous system: immune functional relevance. J Leukoc Biol. 2005;78:1192–1197. - PubMed
-
- Carrier EJ, Kearn CS, Barkmeier AJ, Breese NM, Yang W, Nithipatikom K, Pfister SL, Campbell WB, Hillard CJ. Cultured rat microglial cells synthesize the endocannabinoids 1-arachidonylglycerol, which increases proleferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

