Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK
- PMID: 21256905
- PMCID: PMC3094601
- DOI: 10.1016/j.jhep.2010.12.034
Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK
Abstract
Background & aims: Mechanisms underlying synergistic liver injury caused by alcohol and obesity are not clear. We have produced a mouse model of synergistic steatohepatitis by recapitulating the natural history of the synergism seen in patients for mechanistic studies.
Methods: Moderate obesity was induced in mice by 170% overnutrition in calories using intragastric overfeeding of high fat diet. Alcohol (low or high dose) was then co-administrated to determine its effects.
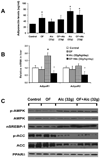
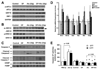
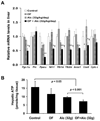
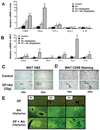
Results: Moderate obesity plus alcohol intake causes synergistic steatohepatitis in an alcohol dose-dependent manner. A heightened synergism is observed when a high alcohol dose (32g/kg/d) is used, resulting in plasma ALT reaching 392±28U/L, severe steatohepatitis with pericellular fibrosis, marked M1 macrophage activation, a 40-fold induction of iNos, and intensified nitrosative stress in the liver. Hepatic expression of genes for mitochondrial biogenesis and metabolism are significantly downregulated, and hepatic ATP level is decreased. Synergistic ER stress evident by elevated XBP-1, GRP78 and CHOP is accompanied by hyperhomocysteinemia. Despite increased caspase 3/7 cleavage, their activities are decreased in a redox-dependent manner. Neither increased PARP cleavage nor TUNEL positive hepatocytes are found, suggesting a shift of apoptosis to necrosis. Surprisingly, the synergism mice have increased plasma adiponectin and hepatic p-AMPK, but adiponectin resistance is shown downstream of p-AMPK.
Conclusions: Nitrosative stress mediated by M1 macrophage activation, adiponectin resistance, and accentuated ER and mitochondrial stress underlie potential mechanisms for synergistic steatohepatitis caused by moderate obesity and alcohol.
Copyright © 2011 European Association for the Study of the Liver. Published by Elsevier B.V. All rights reserved.
Figures

References
-
- Ruhl CE, Everhart JE. Joint effects of body weight and alcohol on elevated serum alanine aminotransferase in the United States population. Clin Gastroenterol Hepatol. 2005;3(12):1260–1268. - PubMed
-
- Alatalo PI, Koivisto HM, Hietala JP, Puukka KS, Bloigu R, Niemela OJ. Effect of moderate alcohol consumption on liver enzymes increases with increasing body mass index. Am J Clin Nutr. 2008;88(4):1097–1103. - PubMed
-
- Bunout D, Gattas V, Iturriaga H, Perez C, Pereda T, Ugarte G. Nutritional status of alcoholic patients: it's possible relationship to alcoholic liver damage. Am J Clin Nutr. 1983;38(3):469–473. - PubMed
-
- Naveau S, Giraud V, Borotto E, Aubert A, Capron F, Chaput JC. Excess weight risk factor for alcoholic liver disease. Hepatology. 1997;25(1):108–111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AA007578/AA/NIAAA NIH HHS/United States
- R01 AA018612/AA/NIAAA NIH HHS/United States
- R01 AA014428/AA/NIAAA NIH HHS/United States
- R24 AA012885/AA/NIAAA NIH HHS/United States
- R21 AA016010/AA/NIAAA NIH HHS/United States
- T32AA007578/AA/NIAAA NIH HHS/United States
- R01 DK089185/DK/NIDDK NIH HHS/United States
- R01 AA018846/AA/NIAAA NIH HHS/United States
- 2R01AA014428-06A1/AA/NIAAA NIH HHS/United States
- P50 AA011999/AA/NIAAA NIH HHS/United States
- P50AA011999/AA/NIAAA NIH HHS/United States
- R24AA012885/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

