Effect of nitric oxide on neointimal hyperplasia based on sex and hormone status
- PMID: 21256959
- PMCID: PMC3070831
- DOI: 10.1016/j.freeradbiomed.2011.01.016
Effect of nitric oxide on neointimal hyperplasia based on sex and hormone status
Abstract
Nitric oxide (NO)-based therapies decrease neointimal hyperplasia; however, studies have been performed only in male animal models. Thus, we sought to evaluate the effect of NO on vascular smooth muscle cells (VSMC) in vitro and neointimal hyperplasia in vivo based on sex and hormone status. In hormone-replete medium, male VSMC proliferated at greater rates than female VSMC. In hormone-depleted medium, female VSMC proliferated at greater rates than male VSMC. However, in both hormone environments, NO inhibited proliferation and migration to a greater extent in male compared to female VSMC. These findings correlated with greater G₀/G₁ cell cycle arrest and changes in cell cycle protein expression in male compared to female VSMC after exposure to NO. Next, the rat carotid artery injury model was used to assess the effect of NO on neointimal hyperplasia in vivo. Consistent with the in vitro data, NO was significantly more effective at inhibiting neointimal hyperplasia in hormonally intact males compared to females using weight-based dosing. An increased weight-based dose of NO in females was able to achieve efficacy equal to that in males. Surprisingly, NO was less effective at inhibiting neointimal hyperplasia in castrated animals of both sexes. In conclusion, these data suggest that NO inhibits neointimal hyperplasia more effectively in males compared to females and in hormonally intact compared to castrated rats, indicating that the effects of NO in the vasculature may be sex- and hormone-dependent.
Published by Elsevier Inc.
Figures

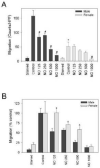
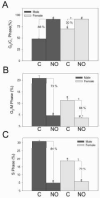
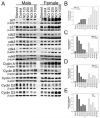



Similar articles
-
Nitric oxide inhibits vascular smooth muscle cell proliferation and neointimal hyperplasia by increasing the ubiquitination and degradation of UbcH10.Cell Biochem Biophys. 2011 Jun;60(1-2):89-97. doi: 10.1007/s12013-011-9179-3. Cell Biochem Biophys. 2011. PMID: 21448667 Free PMC article.
-
Nitric oxide inhibits neointimal hyperplasia following vascular injury via differential, cell-specific modulation of SOD-1 in the arterial wall.Nitric Oxide. 2015 Jan 30;44:8-17. doi: 10.1016/j.niox.2014.10.009. Epub 2014 Nov 7. Nitric Oxide. 2015. PMID: 25460325 Free PMC article.
-
Nitric oxide and nanotechnology: a novel approach to inhibit neointimal hyperplasia.J Vasc Surg. 2008 Jan;47(1):173-82. doi: 10.1016/j.jvs.2007.09.005. J Vasc Surg. 2008. PMID: 18178471 Free PMC article.
-
Heightened efficacy of nitric oxide-based therapies in type II diabetes mellitus and metabolic syndrome.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2388-98. doi: 10.1152/ajpheart.00185.2008. Epub 2008 Oct 17. Am J Physiol Heart Circ Physiol. 2008. PMID: 18931034 Free PMC article.
-
Periadventitial adipose tissue modulates the effect of PROLI/NO on neointimal hyperplasia.J Surg Res. 2016 Oct;205(2):440-445. doi: 10.1016/j.jss.2016.06.074. Epub 2016 Jul 5. J Surg Res. 2016. PMID: 27664894 Free PMC article.
Cited by
-
Rutaecarpine Inhibits Intimal Hyperplasia in A Balloon-Injured Rat Artery Model.Chin J Integr Med. 2018 Jun;24(6):429-435. doi: 10.1007/s11655-017-2900-3. Epub 2017 Sep 1. Chin J Integr Med. 2018. PMID: 28861806
-
The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex.J Surg Res. 2012 Mar;173(1):e1-10. doi: 10.1016/j.jss.2011.09.021. Epub 2011 Oct 8. J Surg Res. 2012. PMID: 22099601 Free PMC article.
-
Cell sex affects extracellular matrix protein expression and proliferation of smooth muscle progenitor cells derived from human pluripotent stem cells.Stem Cell Res Ther. 2017 Jul 4;8(1):156. doi: 10.1186/s13287-017-0606-2. Stem Cell Res Ther. 2017. PMID: 28676082 Free PMC article.
-
Hydroxysafflor Yellow A inhibits the viability and migration of vascular smooth muscle cells induced by serum from rats with chronic renal failure via inactivation of the PI3K/Akt signaling pathway.Exp Ther Med. 2021 Aug;22(2):850. doi: 10.3892/etm.2021.10282. Epub 2021 Jun 8. Exp Ther Med. 2021. PMID: 34149896 Free PMC article.
-
Sex-related differences in matrix remodeling and early osteogenic markers in aortic valvular interstitial cells.Heart Vessels. 2017 Feb;32(2):217-228. doi: 10.1007/s00380-016-0909-8. Epub 2016 Oct 19. Heart Vessels. 2017. PMID: 27761653
References
-
- Kibbe M, Billiar T, Tzeng E. Inducible nitric oxide synthase and vascular injury. Cardiovasc Res. 1999 Aug 15;43(3):650–7. - PubMed
-
- Ahanchi SS, Tsihlis ND, Kibbe MR. The role of nitric oxide in the pathophysiology of intimal hyperplasia. J Vasc Surg. 2007 Jun;45(Suppl A):A64–A73. A64-73. - PubMed
-
- Kibbe MR, Li J, Nie S, Watkins SC, Lizonova A, Kovesdi I, et al. Inducible nitric oxide synthase (iNOS) expression upregulates p21 and inhibits vascular smooth muscle cell proliferation through p42/44 mitogen-activated protein kinase activation and independent of p53 and cyclic guanosine monophosphate. J Vasc Surg. 2000 Jun;31(6):1214–28. - PubMed
-
- Lloyd-Jones D, Adams R, Carnethon M, De SG, Ferguson TB, Flegal K, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 Dec 15; - PubMed
-
- Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991 Apr 10;265(14):1861–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

