Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803
- PMID: 21257306
- PMCID: PMC3073413
- DOI: 10.1016/j.ejca.2010.12.015
Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803
Abstract
Background: The ACCENT group previously established disease-free survival (DFS) with 2 or 3 years median follow-up to predict 5 year overall survival (5 year OS) in stage II and III colon cancer. ACCENT further proposed (1) a stronger association between DFS and OS in stage III than II, and (2) 6 or 7 years necessary to demonstrate DFS/OS surrogacy in recent trials. The relationship between end-points in trials with oral fluoropyrimidines, oxaliplatin and irinotecan is unknown.
Methods: Associations between the treatment effect hazard ratios (HRs) on 2 and 3 years DFS, and 5 and 6 years OS were examined in 6 phase III trials not included in prior analyses from 1997 to 2002. Individual data for 12,676 patients were analysed; two trials each tested oxaliplatin, irinotecan and oral treatment versus 5-FU/LV.
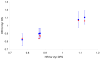
Findings: Overall association between 2/3 year DFS and 5/6 year OS HRs was modest to poor (simple R² measures: 0.58-0.76, model-based R²: 0.17-0.49). In stage III patients, the association increased (model-based R² ≥ 0.79). Observed treatment effects on 2 year DFS accurately 5/6 year OS effects overall and in stage III patients.
Interpretation: In recent trials of cytotoxic chemotherapy, 2 or 3 years DFS HRs are highly predictive of 5 and 6 years OS HRs in stage III but not stage II patients. In all patients the DFS/OS association is stronger for 6 year OS, thus at least 6 year follow-up is recommended to assess OS benefit. These data support DFS as the primary end-point for stage III colon cancer trials testing cytotoxic agents.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures




References
-
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. - PubMed
-
- Sargent DJ, Sobrero A, Grothey A, O’Connell MJ, Buyse M, Andre T, Zheng Y, Green E, Labianca R, O’Callaghan C, Seitz JF, Francini G, Haller D, Yothers G, Goldberg R, de Gramont A. Evidence for cure by adjuvant therapy in colon cancer: Observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009 Feb. 2027(6):872–877. - PMC - PubMed
-
- Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005 Dec 1;23(34):8664–70. - PubMed
-
- Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ, Francini G, Grothey A, O’Connell M, Catalano PJ, Kerr D, Green E, Wieand HS, Goldberg RM, de Gramont A. End points for colon cancer adjuvant trials: Observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007 Oct. 1025(29):4569–4574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

