Accumulation and distribution of α-synuclein and ubiquitin in the CNS of Gaucher disease mouse models
- PMID: 21257328
- PMCID: PMC3059359
- DOI: 10.1016/j.ymgme.2010.12.014
Accumulation and distribution of α-synuclein and ubiquitin in the CNS of Gaucher disease mouse models
Abstract
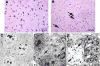
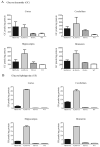
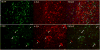
Gaucher disease, a prevalent lysosomal storage disease, is caused by insufficient activity of acid β-glucosidase (GCase) and resultant glucosylceramide accumulation. Recently in Parkinson disease (PD) patients, heterozygous mutations in GCase have been associated with earlier onset and more progressive PD. To understand the pathogenic relationships between GCase variants and Parkinsonism, α-synuclein and ubiquitin distributions and levels in the brains of several mouse models containing GCase variants were evaluated by immunohistochemistry. Progressive α-synuclein and ubiquitin aggregate accumulations were observed in the cortex, hippocampus, basal ganglia, brainstem, and some cerebellar regions between 4 and 24 weeks in mice that were homozygous for GCase [D409H (9H) or V394L (4L)] variants and also had a prosaposin hypomorphic (PS-NA) transgene. In 4L/PS-NA and 9H/PS-NA mice, this was coincident with progressive neurological manifestations and brain glucosylceramide accumulation. Ultrastructural studies showed electron dense inclusion bodies in neurons and axons of 9H/PS-NA brains. α-synuclein aggregates were also observed in ventricular, brainstem, and cerebellar regions of older mice (>42-weeks) with the GCase variant (D409H/D409H) without overt neurological disease. In a chemically induced GCase deficiency, α-synuclein aggregates and glucosylceramide accumulation also occurred. These studies demonstrate a relationship between glucosylceramide accumulation and α-synuclein aggregates, and implicate glucosylceramide accumulation as risk factor for the α-synucleinopathies.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures









References
-
- Beutler E, Grabowski GA. Gaucher Disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2001. pp. 3635–3668.
-
- Grabowski GA, Saal HM, Wenstrup RJ, Barton NW. Gaucher disease: a prototype for molecular medicine. Crit Rev Oncol Hematol. 1996;23:25–55. - PubMed
-
- Zimran A, Kay A, Gelbart T, Garver P, Thurston D, Saven A, Beutler E. Gaucher disease. Clinical, laboratory, radiologic, and genetic features of 53 patients. Medicine (Baltimore) 1992;71:337–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

