Challenges and opportunities for next-generation intracortically based neural prostheses
- PMID: 21257365
- PMCID: PMC3150197
- DOI: 10.1109/TBME.2011.2107553
Challenges and opportunities for next-generation intracortically based neural prostheses
Abstract
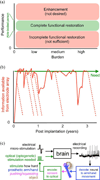
Neural prosthetic systems aim to help disabled patients by translating neural signals from the brain into control signals for guiding computer cursors, prosthetic arms, and other assistive devices. Intracortical electrode arrays measure action potentials and local field potentials from individual neurons, or small populations of neurons, in the motor cortices and can provide considerable information for controlling prostheses. Despite several compelling proof-of-concept laboratory animal experiments and an initial human clinical trial, at least three key challenges remain which, if left unaddressed, may hamper the translation of these systems into widespread clinical use. We review these challenges: achieving able-bodied levels of performance across tasks and across environments, achieving robustness across multiple decades, and restoring able-bodied quality proprioception and somatosensation. We also describe some emerging opportunities for meeting these challenges. If these challenges can be largely or fully met, intracortically based neural prostheses may achieve true clinical viability and help increasing numbers of disabled patients.
© 2011 IEEE
Figures


References
-
- Anderson KD. Targeting recovery: Priorities of the spinal cord-injured population. J. Neurotrauma. 2004;vol. 21:1371–1383. - PubMed
-
- Scherberger H. Neural control of motor prostheses. Curr. Opin. Neurobiol. 2009;vol. 19:629–633. - PubMed
-
- Nicolelis MAL, Lebedev MA. Principles of neural ensemble physiology underlying the operation of brain–machine interfaces. Nat. Rev. Neurosci. 2009;vol. 10:530–540. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

