Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury
- PMID: 21257713
- PMCID: PMC3058708
- DOI: 10.1096/fj.10-160051
Noninvasive molecular imaging reveals role of PAF in leukocyte-endothelial interaction in LPS-induced ocular vascular injury
Abstract
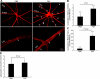
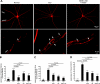
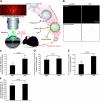
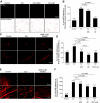
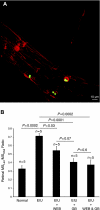
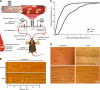
Uveitis is a systemic immune disease and a common cause of blindness. The eye is an ideal organ for light-based imaging of molecular events underlying vascular and immune diseases. The phospholipid platelet-activating factor (PAF) is an important mediator of inflammation, the action of which in endothelial and immune cells in vivo is not well understood. The purpose of this study was to investigate the role of PAF in endothelial injury in uveitis. Here, we use our recently introduced in vivo molecular imaging approach in combination with the PAF inhibitors WEB 2086 (WEB) and ginkgolide B (GB). The differential inhibitory effects of WEB and GB in reducing LPS-induced endothelial injury in the choroid indicate an important role for PAF-like lipids, which might not require the PAF receptor for their signaling. P-selectin glycoprotein ligand-1-mediated rolling of mouse leukocytes on immobilized P-selectin in our autoperfused microflow chamber assay revealed a significant reduction in rolling velocity on the cells' contact with PAF. Rolling cells that came in contact with PAF rapidly assumed morphological signs of cell activation, indicating that activation during rolling does not require integrins. Our results show a key role for PAF in mediating endothelial and leukocyte activation in acute ocular inflammation. Our in vivo molecular imaging provides a detailed view of cellular and molecular events in the complex physiological setting.
Figures
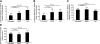
References
-
- Suzuma K., Mandai M., Kogishi J., Tojo S. J., Honda Y., Yoshimura N. (1997) Role of P-selectin in endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 38, 1610–1618 - PubMed
-
- Miyamoto K., Ogura Y., Hamada M., Nishiwaki H., Hiroshiba N., Honda Y. (1996) In vivo quantification of leukocyte behavior in the retina during endotoxin-induced uveitis. Invest. Ophthalmol. Vis. Sci. 37, 2708–2715 - PubMed
-
- Norman K. E., Moore K. L., McEver R. P., Ley K. (1995) Leukocyte rolling in vivo is mediated by P-selectin glycoprotein ligand-1. Blood 86, 4417–4421 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

