SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis
- PMID: 21257720
- PMCID: PMC3060291
- DOI: 10.1158/1078-0432.CCR-10-2476
SST0001, a chemically modified heparin, inhibits myeloma growth and angiogenesis via disruption of the heparanase/syndecan-1 axis
Abstract
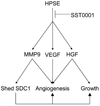
Purpose: Heparanase promotes myeloma growth, dissemination, and angiogenesis through modulation of the tumor microenvironment, thus highlighting the potential of therapeutically targeting this enzyme. SST0001, a nonanticoagulant heparin with antiheparanase activity, was examined for its inhibition of myeloma tumor growth in vivo and for its mechanism of action.
Experimental design: The ability of SST0001 to inhibit growth of myeloma tumors was assessed using multiple animal models and a diverse panel of human and murine myeloma cell lines. To investigate the mechanism of action of SST0001, pharmacodynamic markers of angiogenesis, heparanase activity, and pathways downstream of heparanase were monitored. The potential use of SST0001 as part of a combination therapy was also evaluated in vivo.
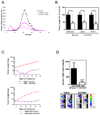
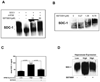
Results: SST0001 effectively inhibited myeloma growth in vivo, even when confronted with an aggressively growing tumor within human bone. In addition, SST0001 treatment causes changes within tumors consistent with the compound's ability to inhibit heparanase, including downregulation of HGF, VEGF, and MMP-9 expression and suppressed angiogenesis. SST0001 also diminishes heparanase-induced shedding of syndecan-1, a heparan sulfate proteoglycan known to be a potent promoter of myeloma growth. SST0001 inhibited the heparanase-mediated degradation of syndecan-1 heparan sulfate chains, thus confirming the antiheparanase activity of this compound. In combination with dexamethasone, SST0001 blocked tumor growth in vivo presumably through dual targeting of the tumor and its microenvironment.
Conclusions: These results provide mechanistic insight into the antitumor action of SST0001 and validate its use as a novel therapeutic tool for treating multiple myeloma.
©2011 AACR.
Conflict of interest statement
JPR, VCR, YR, MT and YY declare no conflict of interest.
Figures


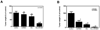
References
-
- Bergsagel D. The incidence and epidemiology of plasma cell neoplasms. Stem Cells. 1995;13 Suppl 2:1–9. - PubMed
-
- Laubach JP, Richardson PG, Anderson KC. The evolution and impact of therapy in multiple myeloma. Med Oncol. 27 Suppl 1:S1–S6. - PubMed
-
- Anderson KC. Targeted therapy of multiple myeloma based upon tumor-microenvironmental interactions. Exp Hematol. 2007;35:155–162. - PubMed
-
- Kelly T, Miao HQ, Yang Y, et al. High heparanase activity in multiple myeloma is associated with elevated microvessel density. Cancer Res. 2003;63:8749–8756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

