Unusual heme-binding PAS domain from YybT family proteins
- PMID: 21257773
- PMCID: PMC3067658
- DOI: 10.1128/JB.01364-10
Unusual heme-binding PAS domain from YybT family proteins
Abstract
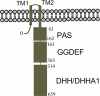
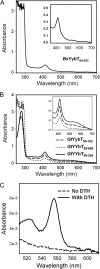
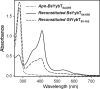
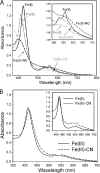
YybT family proteins (COG3887) are functionally unknown proteins that are widely distributed among the firmicutes, including the human pathogens Staphylococcus aureus and Listeria monocytogenes. Recent studies suggested that YybT family proteins are crucial for the in vivo survival of bacterial pathogens during host infection. YybT family proteins contain an N-terminal domain that shares minimum sequence homology with Per-ARNT-Sim (PAS) domains. Despite the lack of an apparent residue for heme coordination, the putative PAS domains of BsYybT and GtYybT, two representative members of the YybT family proteins from Bacillus subtilis and Geobacillus thermodenitrificans, respectively, are found to bind b-type heme with 1:1 stoichiometry. Heme binding suppresses the catalytic activity of the DHH/DHHA1 phosphodiesterase domain and the degenerate GGDEF domain. Absorption spectroscopic studies indicate that YybT proteins do not form stable oxyferrous complexes due to the rapid oxidation of the ferrous iron upon O(2) binding. The ferrous heme, however, forms a hexacoordinated complex with carbon monoxide (CO) and a pentacoordinated complex with nitric oxide (NO). The coordination of NO, but not CO, to the heme stimulates the phosphodiesterase activity. These results suggest that YybT family proteins function as stress-signaling proteins for monitoring cellular heme or the NO level by using a heme-binding PAS domain that features an unconventional heme coordination environment.
Figures
References
-
- Berry, E. A., and B. L. Trumpower. 1987. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Anal. Biochem. 161:1-15. - PubMed
-
- Castiglione, N., S. Rinaldo, G. Giardina, and F. Cutruzzola. 2009. The transcription factor DNR from Pseudomonas aeruginosa specifically requires nitric oxide and haem for the activation of a target promoter in Escherichia coli. Microbiology 155:2838-2844. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

