Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain
- PMID: 21257908
- PMCID: PMC3038698
- DOI: 10.1073/pnas.1014335108
Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain
Abstract
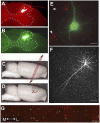
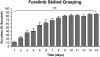
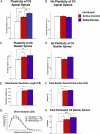
Cortical networks undergo adaptations during learning, including increases in dendritic complexity and spines. We hypothesized that structural elaborations during learning are restricted to discrete subsets of cells preferentially activated by, and relevant to, novel experience. Accordingly, we examined corticospinal motor neurons segregated on the basis of their distinct descending projection patterns, and their contribution to specific aspects of motor control during a forelimb skilled grasping task in adult rats. Learning-mediated structural adaptations, including extensive expansions of spine density and dendritic complexity, were restricted solely to neurons associated with control of distal forelimb musculature required for skilled grasping; neurons associated with control of proximal musculature were unchanged by the experience. We further found that distal forelimb-projecting and proximal forelimb-projecting neurons are intermingled within motor cortex, and that this distribution does not change as a function of skill acquisition. These findings indicate that representations of novel experience in the adult motor cortex are associated with selective structural expansion in networks of functionally related, active neurons that are distributed across a single cortical domain. These results identify a distinct parcellation of cortical resources in support of learning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Biphasic plasticity of dendritic fields in layer V motor neurons in response to motor learning.Neurobiol Learn Mem. 2015 Nov;125:189-94. doi: 10.1016/j.nlm.2015.08.009. Epub 2015 Aug 28. Neurobiol Learn Mem. 2015. PMID: 26318492
-
Rehabilitation drives enhancement of neuronal structure in functionally relevant neuronal subsets.Proc Natl Acad Sci U S A. 2016 Mar 8;113(10):2750-5. doi: 10.1073/pnas.1514682113. Epub 2016 Feb 22. Proc Natl Acad Sci U S A. 2016. PMID: 26903653 Free PMC article.
-
Selective plasticity of layer 2/3 inputs onto distal forelimb controlling layer 5 corticospinal neurons with skilled grasp motor training.Cell Rep. 2024 Apr 23;43(4):113986. doi: 10.1016/j.celrep.2024.113986. Epub 2024 Apr 9. Cell Rep. 2024. PMID: 38598336
-
Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord.J Appl Physiol (1985). 2006 Dec;101(6):1776-82. doi: 10.1152/japplphysiol.00515.2006. Epub 2006 Sep 7. J Appl Physiol (1985). 2006. PMID: 16959909 Review.
-
Circuit changes in motor cortex during motor skill learning.Neuroscience. 2018 Jan 1;368:283-297. doi: 10.1016/j.neuroscience.2017.09.010. Epub 2017 Sep 14. Neuroscience. 2018. PMID: 28918262 Free PMC article. Review.
Cited by
-
Cortical plasticity during motor learning and recovery after ischemic stroke.Neural Plast. 2011;2011:871296. doi: 10.1155/2011/871296. Epub 2011 Oct 26. Neural Plast. 2011. PMID: 22135758 Free PMC article. Review.
-
Clinical neuroscience and neurotechnology: An amazing symbiosis.iScience. 2022 Sep 16;25(10):105124. doi: 10.1016/j.isci.2022.105124. eCollection 2022 Oct 21. iScience. 2022. PMID: 36193050 Free PMC article. Review.
-
Sleep-Dependent Reactivation of Ensembles in Motor Cortex Promotes Skill Consolidation.PLoS Biol. 2015 Sep 18;13(9):e1002263. doi: 10.1371/journal.pbio.1002263. eCollection 2015. PLoS Biol. 2015. PMID: 26382320 Free PMC article.
-
Thalamocortical Projections onto Behaviorally Relevant Neurons Exhibit Plasticity during Adult Motor Learning.Neuron. 2016 Mar 16;89(6):1173-1179. doi: 10.1016/j.neuron.2016.02.001. Epub 2016 Mar 3. Neuron. 2016. PMID: 26948893 Free PMC article.
-
Corticothalamic neurons in motor cortex have a permissive role in motor execution.Nat Commun. 2025 May 21;16(1):4735. doi: 10.1038/s41467-025-59954-1. Nat Commun. 2025. PMID: 40399266 Free PMC article.
References
-
- Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–314. - PubMed
-
- Withers GS, Greenough WT. Reach training selectively alters dendritic branching in subpopulations of layer II-III pyramids in rat motor-somatosensory forelimb cortex. Neuropsychologia. 1989;27:61–69. - PubMed
-
- Adkins DL, Bury SD, Jones TA. Laminar-dependent dendritic spine alterations in the motor cortex of adult rats following callosal transection and forced forelimb use. Neurobiol Learn Mem. 2002;78:35–52. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

