Cytoprotective-selective activated protein C attenuates Pseudomonas aeruginosa-induced lung injury in mice
- PMID: 21257925
- PMCID: PMC3175583
- DOI: 10.1165/rcmb.2010-0397OC
Cytoprotective-selective activated protein C attenuates Pseudomonas aeruginosa-induced lung injury in mice
Abstract
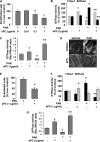
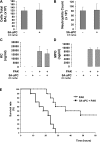
Inhibition of the small GTPase RhoA attenuates the development of pulmonary edema and restores positive alveolar fluid clearance in a murine model of Pseudomonas aeruginosa pneumonia. Activated protein C (aPC) blocks the development of an unfavorably low ratio of small GTPase Rac1/RhoA activity in lung endothelium through endothelial protein C receptor (EPCR)/protease-activated receptor-1 (PAR-1)-dependent signaling mechanisms that include transactivating the sphingosine-1-phosphate (S1P) pathway. However, whether aPC's cytoprotective effects can attenuate the development of pulmonary edema and death associated with P. aeruginosa pneumonia in mice remains unknown. Thus, we determined whether the normalization of a depressed ratio of activated Rac1/RhoA by aPC would attenuate the P. aeruginosa-mediated increase in protein permeability across lung endothelial and alveolar epithelial barriers. Pretreatment with aPC significantly reduced P. aeruginosa-induced increases in paracellular permeability across pulmonary endothelial cell and alveolar epithelial monolayers via an inhibition of RhoA activation and a promotion of Rac1 activation that required the EPCR-PAR-1 and S1P pathways. Furthermore, pretreatment with aPC attenuated the development of pulmonary edema in a murine model of P. aeruginosa pneumonia. Finally, a cytoprotective-selective aPC mutant, aPC-5A, which lacks most of aPC's anticoagulant activity, reproduced the protective effect of wild-type aPC by attenuating the development of pulmonary edema and decreasing mortality in a murine model of P. aeruginosa pneumonia. Taken together, these results demonstrate a critical role for the cytoprotective activities of aPC in attenuating P. aeruginosa-induced lung vascular permeability and mortality, suggesting that cytoprotective-selective aPC-5A with diminished bleeding risks could attenuate the lung damage caused by P. aeruginosa in critically ill patients.
Figures





References
-
- Rello J, Allegri C, Rodriguez A, Vidaur L, Sirgo G, Gomez F, Agbaht K, Pobo A, Diaz E. Risk factors for ventilator-associated pneumonia by Pseudomonas aeruginosa in presence of recent antibiotic exposure. Anesthesiology 2006;105:709–714 - PubMed
-
- Rello J, Rue M, Jubert P, Muses G, Sonora R, Valles J, Niederman MS. Survival in patients with nosocomial pneumonia: impact of the severity of illness and the etiologic agent. Crit Care Med 1997;25:1862–1867 - PubMed
-
- Gunther A, Mosavi P, Heinemann S, Ruppert C, Muth H, Markart P, Grimminger F, Walmrath D, Temmesfeld-Wollbruck B, Seeger W. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia: comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med 2000;161:454–462 - PubMed
-
- Mosnier LO, Zlokovic BV, Griffin JH. The cytoprotective protein C pathway. Blood 2007;109:3161–3172 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

