A novel human IgA monoclonal antibody protects against tuberculosis
- PMID: 21257971
- PMCID: PMC3115510
- DOI: 10.4049/jimmunol.1003189
A novel human IgA monoclonal antibody protects against tuberculosis
Abstract
Abs have been shown to be protective in passive immunotherapy of tuberculous infection using mouse experimental models. In this study, we report on the properties of a novel human IgA1, constructed using a single-chain variable fragment clone (2E9), selected from an Ab phage library. The purified Ab monomer revealed high binding affinities for the mycobacterial α-crystallin Ag and for the human FcαRI (CD89) IgA receptor. Intranasal inoculations with 2E9IgA1 and recombinant mouse IFN-γ significantly inhibited pulmonary H37Rv infection in mice transgenic for human CD89 but not in CD89-negative littermate controls, suggesting that binding to CD89 was necessary for the IgA-imparted passive protection. 2E9IgA1 added to human whole-blood or monocyte cultures inhibited luciferase-tagged H37Rv infection although not for all tested blood donors. Inhibition by 2E9IgA1 was synergistic with human rIFN-γ in cultures of purified human monocytes but not in whole-blood cultures. The demonstration of the mandatory role of FcαRI (CD89) for human IgA-mediated protection is important for understanding of the mechanisms involved and also for translation of this approach toward development of passive immunotherapy of tuberculosis.
Figures


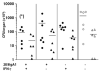
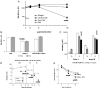
References
-
- Nunn P, Williams B, Floyd K, Dye C, Elzinga G, Raviglione M. Tuberculosis control in the era of HIV. Nat. Rev. Immunol. 2005;5:819–826. - PubMed
-
- Pethe K, Alonso S, Biet F, Delogu G, Brennan MJ, Locht C, Menozzi FD. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

