Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children
- PMID: 21258105
- PMCID: PMC3060899
- DOI: 10.1093/cid/ciq161
Induction therapy with protease-inhibitors modifies the effect of nevirapine resistance on virologic response to nevirapine-based HAART in children
Abstract
Background: Nevirapine resistance after failed prophylaxis to prevent mother-to-child human immunodeficiency virus (HIV) transmission can compromise subsequent nevirapine-based highly active antiretroviral therapy (HAART).
Methods: Nevirapine-exposed children who achieved virologic suppression with lopinavir/ritonavir-based induction HAART before switch to nevirapine-based HAART or who continued the lopinavir/ritonavir regimen were studied. Nevirapine-resistant HIV was quantified (≥ 1% frequency) in plasma before therapy and archived in peripheral blood mononuclear cells after induction HAART with ultradeep pyrosequencing. The primary endpoint was virologic failure (confirmed viremia ≥ 1000 copies/mL by 52 weeks) on nevirapine-based HAART, and Receiver operating characteristic analysis identified threshold levels of resistance associated with failure.
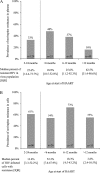
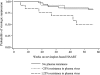
Results: Nevirapine resistance mutations were detected in plasma at a median frequency of 25.6% in 41 (33%) of 124 children starting HAART at median 9 months of age. After a median nine months of induction HAART, nevirapine-resistant HIV remained archived in cells in 59 (61%) of 96 children (median 13.6% of cells). The threshold frequency of nevirapine resistance in plasma most predictive of virologic failure on nevirapine-based HAART was 25%. Children maintaining resistance before therapy at or above this threshold frequency had a 3.5 fold higher risk of failure (95% confidence interval, 1.1-10.8) than children without detectable plasma resistance. In contrast, virologic failure was not independently associated with age, resistance in plasma below 25% frequencies, or archived in cells.
Conclusions: Virologic suppression with lopinavir/ritonavir-based HAART in nevirapine-exposed children raises the threshold level of resistance at which reuse of nevirapine-based therapy is compromised. Standard genotyping may allow identification of children likely to benefit from an induction-switch approach.
Figures

Similar articles
-
Rapid development of antiretroviral drug resistance mutations in HIV-infected children less than two years of age initiating protease inhibitor-based therapy in South Africa.AIDS Res Hum Retroviruses. 2011 Sep;27(9):945-56. doi: 10.1089/aid.2010.0205. Epub 2011 Mar 23. AIDS Res Hum Retroviruses. 2011. PMID: 21345162 Free PMC article.
-
Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial.JAMA. 2010 Sep 8;304(10):1082-90. doi: 10.1001/jama.2010.1278. JAMA. 2010. PMID: 20823434 Free PMC article. Clinical Trial.
-
Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial.Lancet Infect Dis. 2012 Jul;12(7):521-30. doi: 10.1016/S1473-3099(12)70051-8. Epub 2012 Mar 16. Lancet Infect Dis. 2012. PMID: 22424722 Free PMC article. Clinical Trial.
-
Study of the impact of HIV genotypic drug resistance testing on therapy efficacy.Verh K Acad Geneeskd Belg. 2001;63(5):447-73. Verh K Acad Geneeskd Belg. 2001. PMID: 11813503 Review.
-
Lopinavir/ritonavir: a review of its use in the management of HIV infection.Drugs. 2003;63(8):769-802. doi: 10.2165/00003495-200363080-00004. Drugs. 2003. PMID: 12662125 Review.
Cited by
-
PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8+ T cells in mice.J Clin Invest. 2013 Jun;123(6):2629-42. doi: 10.1172/JCI64704. Epub 2013 May 1. J Clin Invest. 2013. PMID: 23635778 Free PMC article.
-
Antiretroviral treatment in HIV-1 infected pediatric patients: focus on efavirenz.Pediatric Health Med Ther. 2014 May 29;5:29-42. doi: 10.2147/PHMT.S47794. Pediatric Health Med Ther. 2014. PMID: 25937791 Free PMC article.
-
Challenges and opportunities in estimating viral genetic diversity from next-generation sequencing data.Front Microbiol. 2012 Sep 11;3:329. doi: 10.3389/fmicb.2012.00329. eCollection 2012. Front Microbiol. 2012. PMID: 22973268 Free PMC article.
-
World Health Organization generic protocol to assess drug-resistant HIV among children <18 months of age and newly diagnosed with HIV in resource-limited countries.Clin Infect Dis. 2012 May;54 Suppl 4(Suppl 4):S254-60. doi: 10.1093/cid/cis003. Clin Infect Dis. 2012. PMID: 22544184 Free PMC article.
-
Concordance between allele-specific PCR and ultra-deep pyrosequencing for the detection of HIV-1 non-nucleoside reverse transcriptase inhibitor resistance mutations.J Virol Methods. 2014 Oct;207:182-7. doi: 10.1016/j.jviromet.2014.07.010. Epub 2014 Jul 14. J Virol Methods. 2014. PMID: 25034127 Free PMC article. Clinical Trial.
References
-
- Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. - PubMed
-
- Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–68. - PubMed
-
- Bedri A, Gudetta B, Isehak A, et al. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. - PubMed
-
- Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. - PubMed
-
- Chasela C, Hudgens M, Jamieson D, et al. Paper presented at: 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention. Capetown, South Africa: Both maternal HAART and daily infant nevirapine (NVP) are effective in reducing HIV-1 transmission during breastfeeding in a randomized trial in Malawi: 28 week results of the Breastfeeding, Antiretroviral and Nutrition (BAN) Study. 19–22 July. 2009.

