The plant cell nucleus: a true arena for the fight between plants and pathogens
- PMID: 21258210
- PMCID: PMC3122004
- DOI: 10.4161/psb.6.1.13978
The plant cell nucleus: a true arena for the fight between plants and pathogens
Abstract
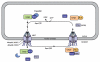
Communication between the cytoplasm and the nucleus is a fundamental feature shared by both plant and animal cells. Cellular factors involved in the transport of macromolecules through the nuclear envelope, including nucleoporins, importins and Ran-GTP related components, are conserved among a variety of eukaryotic systems. Interestingly, mutations in these nuclear components compromise resistance signalling, illustrating the importance of nucleocytoplasmic trafficking in plant innate immunity. Indeed, spatial restriction of defence regulators by the nuclear envelope and stimulus-induced nuclear translocation constitute an important level of defence-associated gene regulation in plants. A significant number of effectors from different microbial pathogens are targeted to the plant cell nucleus. In addition, key host factors, including resistance proteins, immunity components, transcription factors and transcriptional regulators shuttle between the cytoplasm and the nucleus, and their level of nuclear accumulation determines the output of the defence response, further confirming the crucial role played by the nucleus during the interaction between plants and pathogens. Here, we discuss recent findings that situate the nucleus at the frontline of the mutual recognition between plants and invading microbes.
Figures

References
-
- Ausubel FM. Are innate immune signaling pathways in plants and animals conserved? Nat Immunol. 2005;6:973–979. - PubMed
-
- Zipfel C. Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol. 2008;20:10–16. - PubMed
-
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol. 2009;60:379–406. - PubMed
-
- Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–329. - PubMed
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
