Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome
- PMID: 21258343
- PMCID: PMC3045628
- DOI: 10.1038/ng.757
Mutations in lectin complement pathway genes COLEC11 and MASP1 cause 3MC syndrome
Abstract
3MC syndrome has been proposed as a unifying term encompassing the overlapping Carnevale, Mingarelli, Malpuech and Michels syndromes. These rare autosomal recessive disorders exhibit a spectrum of developmental features, including characteristic facial dysmorphism, cleft lip and/or palate, craniosynostosis, learning disability and genital, limb and vesicorenal anomalies. Here we studied 11 families with 3MC syndrome and identified two mutated genes, COLEC11 and MASP1, both of which encode proteins in the lectin complement pathway (collectin kidney 1 (CL-K1) and MASP-1 and MASP-3, respectively). CL-K1 is highly expressed in embryonic murine craniofacial cartilage, heart, bronchi, kidney and vertebral bodies. Zebrafish morphants for either gene develop pigmentary defects and severe craniofacial abnormalities. Finally, we show that CL-K1 serves as a guidance cue for neural crest cell migration. Together, these findings demonstrate a role for complement pathway factors in fundamental developmental processes and in the etiology of 3MC syndrome.
Figures
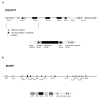
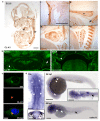


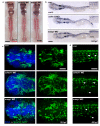

References
-
- Carnevale F, Krajewska G, Fischetto R, Greco MG, Bonvino A. Ptosis of eyelids, strabismus, diastasis recti, hip defect, cryptorchidism, and developmental delay in two sibs. Am J Med Genet. 1989;33:186–9. - PubMed
-
- Malpuech G, Demeocq F, Palcoux JB, Vanlieferinghen P. A previously undescribed autosomal recessive multiple congenital anomalies/mental retardation (MCA/MR) syndrome with growth failure, lip/palate cleft(s), and urogenital anomalies. Am J Med Genet. 1983;16:475–80. - PubMed
-
- Michels VV, Hittner HM, Beaudet AL. A clefting syndrome with ocular anterior chamber defect and lid anomalies. J Pediatr. 1978;93:444–6. - PubMed
-
- Al Kaissi A, et al. Asymmetrical skull, ptosis, hypertelorism, high nasal bridge, clefting, umbilical anomalies, and skeletal anomalies in sibs: is Carnevale syndrome a separate entity? Am J Med Genet A. 2007;143:349–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

