Electrically Triggered Release of a Small Molecule Drug from a Polyelectrolyte Multilayer Coating
- PMID: 21258654
- PMCID: PMC3023308
- DOI: 10.1021/cm102578j
Electrically Triggered Release of a Small Molecule Drug from a Polyelectrolyte Multilayer Coating
Abstract
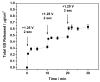
Electrically triggered drug delivery represents an attractive option for actively and remotely controlling the release of a therapeutic from an implantable device (e.g., a "pharmacy-on-a-chip"). Here we report the fabrication of nanoscale thin films that can release precise quantities of a small molecule drug in response to application of a small, anodic electric potential of at least +0.5 V versus Ag/AgCl. Films containing negatively charged Prussian Blue (PB) nanoparticles and positively charged gentamicin, a small hydrophilic antibiotic, were fabricated using layer-by-layer (LbL) assembly. When oxidized, the PB nanoparticles shift from negatively charged to neutral, inducing dissolution of the film. Films with thicknesses in the range 100-500 nm corresponding to drug loadings of 1-4 μg/cm(2) were characterized. We demonstrate control over the drug dosage by tuning the film thickness as well as the magnitude of the applied voltage. Drug release kinetics ranging from triggered burst release to on/off, or pulsatile release, were achieved by applying different electric potential profiles. Finally, the in vitro efficacy of the released drug was confirmed against Staphylococcus aureus bacteria. Given the versatility of an external electrical stimulus and the ability of LbL assembly to conformally coat a variety of substrates regardless of size, shape, or chemical composition, we maintain that electrically controlled release of a drug from an LbL-coated surface could have applications in both implantable medical devices and transdermal drug delivery systems.
Figures









References
Grants and funding
LinkOut - more resources
Full Text Sources
