Repetitive peroxide exposure reveals pleiotropic mitogen-activated protein kinase signaling mechanisms
- PMID: 21258655
- PMCID: PMC3023409
- DOI: 10.1155/2011/636951
Repetitive peroxide exposure reveals pleiotropic mitogen-activated protein kinase signaling mechanisms
Abstract
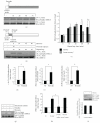
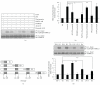
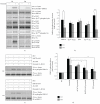
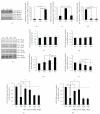
Oxidative stressors such as hydrogen peroxide control the activation of many interconnected signaling systems and are implicated in neurodegenerative disease etiology. Application of hydrogen peroxide to PC12 cells activated multiple tyrosine kinases (c-Src, epidermal growth factor receptor (EGFR), and Pyk2) and the serine-threonine kinase ERK1/2. Peroxide-induced ERK1/2 activation was sensitive to intracellular calcium chelation and EGFR and c-Src kinase inhibition. Acute application and removal of peroxide allowed ERK1/2 activity levels to rapidly subside to basal serum-deprived levels. Using this protocol, we demonstrated that ERK1/2 activation tachyphylaxis developed upon repeated peroxide exposures. This tachyphylaxis was independent of c-Src/Pyk2 tyrosine phosphorylation but was associated with a progressive reduction of peroxide-induced EGFR tyrosine phosphorylation, EGFR interaction with growth factor receptor binding protein 2, and a redistribution of EGFR from the plasma membrane to the cytoplasm. Our data indicates that components of peroxide-induced ERK1/2 cascades are differentially affected by repeated exposures, indicating that oxidative signaling may be contextually variable.
Figures


References
-
- Bumgarner RE, Yeung KY. Methods for the inference of biological pathways and networks. Methods in Molecular Biology. 2009;541:225–245. - PubMed
-
- Maudsley S, Martin B, Luttrell LM. G protein-coupled receptor signaling complexity in neuronal tissue: implications for novel therapeutics. Current Alzheimer Research. 2007;4(1):3–19. - PubMed
-
- Hancock JT. The role of redox mechanisms in cell signalling. Molecular Biotechnology. 2009;43(2):162–166. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

