Spectroscopic and computational characterization of CuII-OOR (R = H or cumyl) complexes bearing a Me6-tren ligand
- PMID: 21258722
- PMCID: PMC3318924
- DOI: 10.1039/c0dt01036g
Spectroscopic and computational characterization of CuII-OOR (R = H or cumyl) complexes bearing a Me6-tren ligand
Abstract
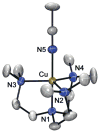
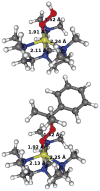
A copper(II)-hydroperoxo complex, [Cu(Me(6)-tren)(OOH)](+) (2), and a copper(ii)-cumylperoxo complex, [Cu(Me(6)-tren)(OOC(CH(3))(2)Ph)](+) (3), were synthesized by reacting [Cu(Me(6)-tren)(CH(3)CN)](2+) (1) with H(2)O(2) and cumyl-OOH, respectively, in the presence of triethylamine. These intermediates, 2 and 3, were successfully characterized by various physicochemical methods such as UV-vis, ESI-MS, resonance Raman and EPR spectroscopies, leading us to propose structures of the Cu(II)-OOR species with a trigonal-bipyramidal geometry. Density functional theory (DFT) calculations provided geometric and electronic configurations of 2 and 3, showing trigonal bipyramidal copper(II)-OOR geometries. These copper(II)-hydroperoxo and -cumylperoxo complexes were inactive in electrophilic and nucleophilic oxidation reactions.
Figures





References
-
- Sheldon RA, Kochi JK. Metal-Catalyzed Oxidations of Organic Compounds. Academic Press; New York: 1981.
-
- Mimoun H. In: The Chemistry of Functional Groups Peroxides. Patai S, editor. Wiley; New York: 1983. pp. 463–482.
-
- Martell AE, Sawyer DT. Oxygen Complexes and Oxygen Activation by Transition Metals. Plenum; New York: 1988.
-
- Spiro TG. Metal ion activation of dioxygen: Metal ions in biology. Wiley-interscience; New York: 1981.
-
- Holm RH, Solomon EI. Chem Rev. 2004;104:347–348. and review articles in the special issue. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

