An eight-week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia
- PMID: 21259004
- PMCID: PMC3061213
- DOI: 10.1007/s00246-011-9885-z
An eight-week trial investigating the efficacy and tolerability of atorvastatin for children and adolescents with heterozygous familial hypercholesterolemia
Abstract
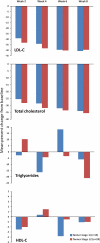
This study aimed to assess the efficacy and tolerability of atorvastatin in Tanner stage (TS) 1 patients ages 6 to 10 years and TS ≥ 2 patients ages 10 to <18 years with genetically confirmed heterozygous familial hypercholesterolemia (HeFH) and a low density lipoprotein cholesterol (LDL-C) level of 4 mmol/l (155 mg/dl) or higher. In this open-label, 8-week study, 15 TS 1 children were treated initially with atorvastatin 5 mg/day and 24 TS ≥ 2 children with 10 mg/day. Doses were doubled at week 4 if the LDL-C target (<3.35 mmol/l [130 mg/dl]) was not achieved. The efficacy variables were the percentage change from baseline in LDL-C, total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), very low density lipoprotein cholesterol (VLDL-C), and apolipoprotein (Apo) A-I and Apo B. Safety evaluations included clinical monitoring, subject-reported adverse events (AEs), vital signs, and clinical laboratory tests. The mean values for LDL-C, TC, VLDL-C, and Apo B decreased by week 2 among all TS 1 and TS ≥ 2 patients, whereas TG, HDL-C, and Apo A-I varied considerably from week to week. After 8 weeks, the mean reduction in LDL-C was -40.7% ± 8.4 for the TS 1 children and -39.7% ± 10.3 for the TS ≥ 2 children. For the TS 1 patients, the mean reductions were -34.1% ± 6.9 for TC and -6.0% ± 32.1 for TG. The corresponding changes for the TS ≥ 2 patients were -35.6% ± 9.5 for TC and -21.1% ± 29.7 for TG. Four patients experienced mild to moderate treatment-related AEs. No serious AEs or discontinuations were reported. Overall, no difference in safety or tolerability was observed between the younger and older cohorts. Across the range of exposures after atorvastatin 5 to 10 mg (TS 1) or atorvastatin 10 to 20 mg (TS ≥ 2) doses for 8 weeks, clinically meaningful reductions in LDL-C, TC, VLDL-C, and Apo were observed with atorvastatin in pediatric patients who had HeFH. Atorvastatin also was well tolerated in this population.
Figures

References
-
- Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/S0735-1097(01)01746-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

