Low-dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine causes inflammatory activation of astrocytes in nuclear factor-κB reporter mice prior to loss of dopaminergic neurons
- PMID: 21259327
- PMCID: PMC6487665
- DOI: 10.1002/jnr.22549
Low-dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine causes inflammatory activation of astrocytes in nuclear factor-κB reporter mice prior to loss of dopaminergic neurons
Abstract
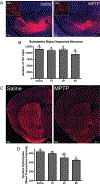
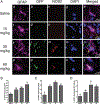
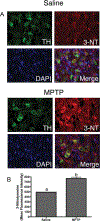
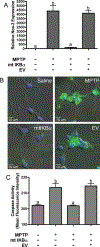
Neuroinflammation is implicated in the progression of numerous disease states of the CNS, but early inflammatory signaling events in glial cells that may predispose neurons to injury are not easily characterized in vivo. To address this question, we exposed transgenic mice expressing a nuclear factor-κB (NF-κB)-driven enhanced green fluorescent protein (EGFP) reporter construct to low doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and examined inflammatory activation of astrocytes in relation to neurobehavioral and neuropathological outcomes. The highest dose of MPTP (60 mg/kg total dose) caused a decrease in locomotor activity and a reduction in stride length. No significant loss of dopaminergic neurons in the substantia nigra was apparent at any dose. In contrast, expression of tyrosine hydroxylase in striatal fibers was reduced at 60 mg/kg MPTP, as were levels of dopamine and DOPAC. Colocalized expression of EGFP and inducible nitric oxide synthase (NOS2) occurred in astrocytes at 30 and 60 mg/kg MPTP and was associated with increased protein nitration in nigral dopaminergic neurons. Inhibition of NF-κB in primary astrocytes by expression of mutant IκBα suppressed expression of NOS2 and protected cocultured neurons from astrocyte-mediated apoptosis. These data indicate that inflammatory activation of astrocytes and enhanced nitrosative stress occurs at low doses of MPTP prior to loss of dopaminergic neurons. NF-κB-mediated expression of NOS2 appears to be a sensitive indicator of neuroinflammation that correlates with MPTP-induced neurochemical and neurobehavioral deficits prior to loss of dopaminergic neurons in the subtantia nigra.
Copyright © 2011 Wiley-Liss, Inc.
Figures


Similar articles
-
Role of nuclear transcription factor kappa B (NF-kappaB) for MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced apoptosis in nigral neurons of mice.Exp Mol Pathol. 2009 Feb;86(1):57-64. doi: 10.1016/j.yexmp.2008.10.004. Epub 2008 Nov 5. Exp Mol Pathol. 2009. PMID: 19027004
-
Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease.J Neuroinflammation. 2010 Nov 23;7:83. doi: 10.1186/1742-2094-7-83. J Neuroinflammation. 2010. PMID: 21092260 Free PMC article.
-
Suppression of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nitric-oxide synthase 2 expression in astrocytes by a novel diindolylmethane analog protects striatal neurons against apoptosis.Mol Pharmacol. 2009 Jan;75(1):35-43. doi: 10.1124/mol.108.050781. Epub 2008 Oct 7. Mol Pharmacol. 2009. PMID: 18840677 Free PMC article.
-
Riluzole (2-amino-6-trifluoromethoxy benzothiazole) attenuates MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in mice.Neurosci Lett. 2001 Oct 12;312(1):50-4. doi: 10.1016/s0304-3940(01)02176-0. Neurosci Lett. 2001. PMID: 11578843
-
Modeling PD pathogenesis in mice: advantages of a chronic MPTP protocol.Parkinsonism Relat Disord. 2008;14 Suppl 2(Suppl 2):S112-5. doi: 10.1016/j.parkreldis.2008.04.012. Epub 2008 Jun 27. Parkinsonism Relat Disord. 2008. PMID: 18585085 Free PMC article. Review.
Cited by
-
Cross-talk between neurons and astrocytes in response to bilirubin: adverse secondary impacts.Neurotox Res. 2014 Jul;26(1):1-15. doi: 10.1007/s12640-013-9427-y. Neurotox Res. 2014. PMID: 24122290
-
Novel para-phenyl substituted diindolylmethanes protect against MPTP neurotoxicity and suppress glial activation in a mouse model of Parkinson's disease.Toxicol Sci. 2015 Feb;143(2):360-73. doi: 10.1093/toxsci/kfu236. Epub 2014 Nov 17. Toxicol Sci. 2015. PMID: 25406165 Free PMC article.
-
The Nurr1 Ligand,1,1-bis(3'-Indolyl)-1-(p-Chlorophenyl)Methane, Modulates Glial Reactivity and Is Neuroprotective in MPTP-Induced Parkinsonism.J Pharmacol Exp Ther. 2018 Jun;365(3):636-651. doi: 10.1124/jpet.117.246389. Epub 2018 Apr 6. J Pharmacol Exp Ther. 2018. PMID: 29626009 Free PMC article.
-
Gastric Enteric Glial Cells: A New Contributor to the Synucleinopathies in the MPTP-Induced Parkinsonism Mouse.Molecules. 2022 Nov 1;27(21):7414. doi: 10.3390/molecules27217414. Molecules. 2022. PMID: 36364248 Free PMC article.
-
The Mechanisms of Traditional Chinese Medicine Underlying the Prevention and Treatment of Parkinson's Disease.Front Pharmacol. 2017 Sep 19;8:634. doi: 10.3389/fphar.2017.00634. eCollection 2017. Front Pharmacol. 2017. PMID: 28970800 Free PMC article. Review.
References
-
- Allen JW, Mutkus LA, Aschner M. 2000. Current protocols in toxicology New York: John Wiley & Sons; 12.4.1–12.4.15.
-
- Aoki E, Yano R, Yokoyama H, Kato H, Araki T. 2009. Role of nuclear transcription factor kappa B (NF-kappaB) for MPTP(1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine)-induced apoptosis in nigral neurons of mice. Exp Mol Pathol 86:57–64. - PubMed
-
- Block ML, Hong JS. 2007. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans 35:1127–1132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

