The Tn antigen-structural simplicity and biological complexity
- PMID: 21259410
- PMCID: PMC7159538
- DOI: 10.1002/anie.201002313
The Tn antigen-structural simplicity and biological complexity
Abstract
Glycoproteins in animal cells contain a variety of glycan structures that are added co- and/or posttranslationally to proteins. Of over 20 different types of sugar-amino acid linkages known, the two major types are N-glycans (Asn-linked) and O-glycans (Ser/Thr-linked). An abnormal mucin-type O-glycan whose expression is associated with cancer and several human disorders is the Tn antigen. It has a relatively simple structure composed of N-acetyl-D-galactosamine with a glycosidic α linkage to serine/threonine residues in glycoproteins (GalNAcα1-O-Ser/Thr), and was one of the first glycoconjugates to be chemically synthesized. The Tn antigen is normally modified by a specific galactosyltransferase (T-synthase) in the Golgi apparatus of cells. Expression of active T-synthase is uniquely dependent on the molecular chaperone Cosmc, which is encoded by a gene on the X chromosome. Expression of the Tn antigen can arise as a consequence of mutations in the genes for T-synthase or Cosmc, or genes affecting other steps of O-glycosylation pathways. Because of the association of the Tn antigen with disease, there is much interest in the development of Tn-based vaccines and other therapeutic approaches based on Tn expression.
Copyright © 2011 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
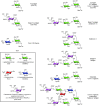

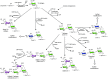


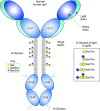
References
-
- Moreau R., Dausset J., Bernard J., Moullec J., Bull. Mem. Soc. Med. Hop. Paris 1957, 73, 569–587. - PubMed
-
- Huebner G., Z. Immunitaetsforsch. Exp. Ther. 1926, 45, 223–248.
-
- Thomsen O., Z. Immunitaetsforsch. Exp. Ther. 1927, 52, 85–107.
-
- Thomsen O., C. R. Seances Soc. Biol. Ses Fil. 1927, 96, 556–558.
-
- Thomsen O., Z. Immunitaetsforsch. Exp. Ther. 1928, 57, 301–319.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

