2-azapinanes: aza analogues of the enantiomeric pinyl carbocation intermediates in pinene biosynthesis
- PMID: 21261271
- PMCID: PMC3415958
- DOI: 10.1021/ol1027893
2-azapinanes: aza analogues of the enantiomeric pinyl carbocation intermediates in pinene biosynthesis
Abstract
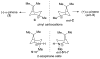
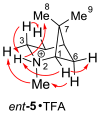
The enantiomeric 2-azapinanes, aza analogues of the pinyl carbocation intermediates in pinene biosynthesis, were synthesized from (-)- and (+)-cis-pinonic acids. The individual reactions in the 5-step sequence were Beckmann rearrangement of the pinonic acid oximes, cyclization to the N-acetyl lactams, hydrolysis to the NH-lactams, N-methylations, and LiAlH(4) reductions. The anti stereochemistry of the N-methyl groups in the salts with respect to the gem-dimethyl bridge was established by NOE measurements and by X-ray diffraction analysis.
Figures



References
-
- Grayson DH. Nat Prod Rep. 2000;17:385–419. - PubMed
- Erman WF. In: In Chemistry of the Monoterpenes Part A. Gassman PG, editor. Vol. 11. Marcel Dekker; New York: 1985. pp. 213–249.
- Erman WF. In: In Chemistry of the Monoterpenes Part B. Gassman PG, editor. Vol. 11. Marcel Dekker; New York: 1985. pp. 929–1033.
-
- Derfer JM, Traynor SG. The Chemistry of Turpentine. In: Zinkel DF, Russell J, editors. Naval Stores: Production, Chemistry, Utilization. Chapter 8 Pulp Chemicals Association; New York: 1989.
-
- Phillips MA, Savage TJ, Croteau R. Arch Biochem Biophys. 1999;372:197–204. - PubMed
-
- Il’ina IV, Volcho KP, Salakhutdinov NF. Russ J Org Chem. 2008;44:1–23.
-
- Ramachandran PV. Aldrichim Acta. 2002;35:23–35.
- Cho BT. Aldrichim Acta. 2002;35:3–16.
- Matteson DS. Stereo-directed Synthesis with Organoboranes. Springer-Verlag; Berlin: 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

