Intermonomer electron transfer between the low-potential b hemes of cytochrome bc₁
- PMID: 21261281
- PMCID: PMC3184928
- DOI: 10.1021/bi101736v
Intermonomer electron transfer between the low-potential b hemes of cytochrome bc₁
Abstract
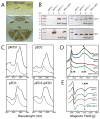
Cytochrome (cyt) bc(1) is a structural dimer with its monomers consisting of the Fe-S protein, cyt b, and cyt c(1) subunits. Its three-dimensional architecture depicts it as a symmetrical homodimer, but the mobility of the head domain of the Fe-S protein indicates that the functional enzyme exists in asymmetrical heterodimeric conformations. Here, we report a new genetic system for studying intra- and intermonomer interactions within the cyt bc(1) using the facultative phototrophic bacterium Rhodobacter capsulatus. The system involves two different sets of independently expressed cyt bc(1) structural genes carried by two plasmids that are coharbored by a cell without its endogenous enzyme. Our results indicate that coexpressed cyt bc(1) subunits were matured, assorted, and assembled in vivo into homo- and heterodimeric enzymes that can bear different mutations in each monomer. Using the system, the occurrence of intermonomer electron transfer between the low-potential b hemes of cyt bc(1) was probed by choosing mutations that perturb electron transfer at the hydroquinone oxidation (Q(o)) and quinone reduction (Q(i)) sites of the enzyme. The data demonstrate that active heterodimeric variants, formed of monomers carrying mutations that abolish only one of the two (Q(o) or Q(i)) active sites of each monomer, are produced, and they support photosynthetic growth of R. capsulatus. Detailed analyses of the physicochemical properties of membranes of these mutants, as well as purified homo- and heterodimeric cyt bc(1) preparations, demonstrated that efficient and productive electron transfer occurs between the low-potential b(L) hemes of the monomers in a heterodimeric enzyme. Overall findings are discussed with respect to intra- and intermonomer interactions that take place during the catalytic turnover of cyt bc(1).
Figures




References
-
- Berry EA, Guergova-Kuras M, Huang LS, Crofts AR. Structure and function of cytochrome bc complexes. Annu Rev Biochem. 2000;69:1005–1075. - PubMed
-
- Mitchell P. Possible molecular mechanisms of the protonmotive function of cytochrome systems. J Theor Biol. 1976;62:327–367. - PubMed
-
- Trumpower BL. The protonmotive Q cycle. Energy transduction by coupling of proton translocation to electron transfer by the cytochrome bc1 complex. J Biol Chem. 1990;265:11409–11412. - PubMed
-
- Daldal F, Davidson E, Cheng S. Isolation of the structural genes for the Rieske Fe-S protein, cytochrome b and cytochrome c1 all components of the ubiquinol: cytochrome c2 oxidoreductase complex of Rhodopseudomonas capsulata. J Mol Biol. 1987;195:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

