Rational methods for the selection of diverse screening compounds
- PMID: 21261294
- PMCID: PMC4765079
- DOI: 10.1021/cb100420r
Rational methods for the selection of diverse screening compounds
Abstract
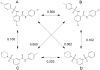
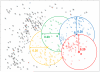
Traditionally a pursuit of large pharmaceutical companies, high-throughput screening assays are becoming increasingly common within academic and government laboratories. This shift has been instrumental in enabling projects that have not been commercially viable, such as chemical probe discovery and screening against high-risk targets. Once an assay has been prepared and validated, it must be fed with screening compounds. Crafting a successful collection of small molecules for screening poses a significant challenge. An optimized collection will minimize false positives while maximizing hit rates of compounds that are amenable to lead generation and optimization. Without due consideration of the relevant protein targets and the downstream screening assays, compound filtering and selection can fail to explore the great extent of chemical diversity and eschew valuable novelty. Herein, we discuss the different factors to be considered and methods that may be employed when assembling a structurally diverse compound collection for screening. Rational methods for selecting diverse chemical libraries are essential for their effective use in high-throughput screens.
Figures

References
-
- Pauling L, Itano H, Singer S, Wells I. Sickle Cell Anemia, a Molecular Disease. Science. 1949;110:543. - PubMed
-
- Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. - PubMed
-
- Kennedy JP, Williams L, Bridges TM, Daniels RN, Weaver D, Lindsley CW. Application of combinatorial chemistry science on modern drug discovery. J Comb Chem. 2008;10:345–354. - PubMed
-
- Spring DR. Chemical genetics to chemical genomics: small molecules offer big insights. Chem Soc Rev. 2005;34:472–482. - PubMed
-
- Kodadek T. Rethinking screening. Nature Chemical Biology. 2010;6:162–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

