Novel anti-inflammatory--pro-resolving mediators and their receptors
- PMID: 21261595
- PMCID: PMC3094721
- DOI: 10.2174/1568026611109060629
Novel anti-inflammatory--pro-resolving mediators and their receptors
Abstract
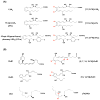
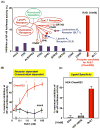
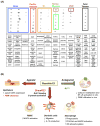
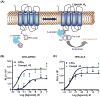
Resolution of inflammation, an actively coordinated program, is essential to maintain host health. It involves effective removal of inflammatory stimuli and the spatio-temporal control of leukocyte trafficking as well as chemical mediator generation. During the active resolution process, new classes of small, local acting endogenous autacoids, namely the lipoxins, D and E series resolvins, (neuro)protectins, and maresins have been identified. These specialized pro-resolving lipid mediators (SPM) prevent excessive inflammation and promote removal of microbes and apoptotic cells, thereby expediting resolution and return to tissue homeostasis. As part of their molecular mechanism, SPM exert their potent actions via activating specific pro-resolving G-protein coupled receptors. Together these SPM and their receptors provide new concepts and opportunities for therapeutics, namely promoting active resolution as opposed to the conventionally used enzyme inhibitors and receptor antagonists. This approach may offer new targets suitable for drug design for treating inflammation related diseases, for the new terrain of resolution pharmacology.
Conflict of interest statement
C.N.S. is inventor on patents assigned to Brigham and Women’s Hospital and Partners Health Care on the composition of matter, uses, and clinical development of anti-inflammatory and proresolving lipid mediators. These are licensed for clinical development. C.N.S. retains founder stock in Resolvyx Pharmaceuticals.
Figures


References
-
- Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. - PMC - PubMed
-
- Serhan CN. Resolution phases of inflammation: novel endogenous anti-inflammatory and pro-resolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. - PubMed
-
- Cotran RS, Kumar V, Collins T, editors. Robbins Pathologic Basis of Disease. W.B. Saunders Co; Philadelphia: 1999. p. 1425.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

