PEGylation of bacteriophages increases blood circulation time and reduces T-helper type 1 immune response
- PMID: 21261844
- PMCID: PMC3815886
- DOI: 10.1111/j.1751-7915.2008.00028.x
PEGylation of bacteriophages increases blood circulation time and reduces T-helper type 1 immune response
Abstract
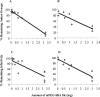
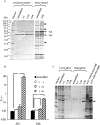
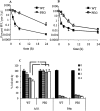
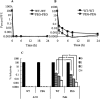
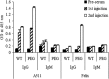
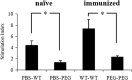
The increasing occurrence of antibiotic-resistant pathogens is of growing concern, and must be counteracted by alternative antimicrobial treatments. Bacteriophages represent the natural enemies of bacteria. However, the strong immune response following application of phages and rapid clearance from the blood stream are hurdles which need to be overcome. Towards our goal to render phages less immunogenic and prolong blood circulation time, we have chemically modified intact bacteriophages by conjugation of the non-immunogenic polymer monomethoxy-polyethylene glycol (mPEG) to virus proteins. As a proof of concept, we have used two different polyvalent and strictly virulent phages of the Myoviridae, representing typical candidates for therapeutical approaches: Felix-O1 (infects Salmonella) and A511 (infects Listeria). Loss of phage infectivity after PEGylation was found to be proportional to the degree of modification, and could be conveniently controlled by adjusting the PEG concentration. When injected into naïve mice, PEGylated phages showed a strong increase in circulation half-life, whereas challenge of immunized mice did not reveal a significant difference. Our results suggest that the prolonged half-life is due to decreased susceptibility to innate immunity as well as avoidance of cellular defence mechanisms. PEGylated viruses elicited significantly reduced levels of T-helper type 1-associated cytokine release (IFN-γ and IL-6), in both naïve and immunized mice. This is the first study demonstrating that PEGylation can increases survival of infective phage by delaying immune responses, and indicates that this approach can increase efficacy of bacteriophage therapy.
© 2008 The Authors.
Figures

References
-
- Abuchowski A., McCoy J.R., Palczuk N.C., Van Es T., Davis F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977a;11:3582–3586. - PubMed
-
- Abuchowski A., Van Es T., Palczuk N.C., Davis F.F. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J Biol Chem. 1977b;11:3578–3581. - PubMed
-
- Adams M.H. Methods of study of bacterial viruses. In: Hershey A.D., editor. Interscience Publishers; 1959. pp. 443–457.
-
- Alemany R., Suzuki K., Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81:2605–2609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

