T-DNA insertion, plasmid rescue and integration analysis in the model mycorrhizal fungus Laccaria bicolor
- PMID: 21261845
- PMCID: PMC3815887
- DOI: 10.1111/j.1751-7915.2008.00029.x
T-DNA insertion, plasmid rescue and integration analysis in the model mycorrhizal fungus Laccaria bicolor
Abstract
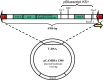
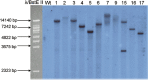
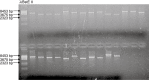
Ectomycorrhiza is a mutualistic symbiosis formed between fine roots of trees and the mycelium of soil fungi. This symbiosis plays a key role in forest ecosystems for the mineral nutrition of trees and the biology of the fungal communities associated. The characterization of genes involved in developmental and metabolic processes is important to understand the complex interactions that control the ectomycorrhizal symbiosis. Agrobacterium-mediated gene transfer (AMT) in fungi is currently opening a new era for fungal research. As whole genome sequences of several fungi are being released studies about T-DNA integration patterns are needed in order to understand the integration mechanisms involved and to evaluate the AMT as an insertional mutagenesis tool for different fungal species. The first genome sequence of a mycorrhizal fungus, the basidiomycete Laccaria bicolor, became public in July 2006. Release of Laccaria genome sequence and the availability of AMT makes this fungus an excellent model for functional genomic studies in ectomycorrhizal research. No data on the integration pattern in Laccaria genome were available, thus we optimized a plasmid rescue approach for this fungus. To this end the transformation vector (pHg/pBSk) was constructed allowing the rescue of the T-DNA right border (RB)-genomic DNA junctions in Escherichia coli. Fifty-one Agrobacterium-transformed fungal strains, picked up at random from a larger collection of T-DNA tagged strains (about 500), were analysed. Sixty-nine per cent were successfully rescued for the RB of which 87% were resolved for genomic integration sequences. Our results demonstrate that the plasmid rescue approach can be used for resolving T-DNA integration sites in Laccaria. The RB was well conserved during transformation of this fungus and the integration analysis showed no clear sequence homology between different genomic sites. Neither obvious sequence similarities were found between these sites and the T-DNA borders indicating non-homologous integration of the transgenes. Majority (75%) of the integrations were located in predicted genes. Agrobacterium-mediated gene transfer is a powerful tool that can be used for functional gene studies in Laccaria and will be helpful along with plasmid rescue in searching for relevant fungal genes involved in the symbiotic process.
© 2008 The Authors.
Figures


References
-
- Alonso J.M., Stepanova A.N., Leisse T.J., Kim C.J., Chen H., Shinn P. Genome‐wide insertional mutagenesis of Arabidopsis thaliana. Science. 2003;301:653–657. et al. - PubMed
-
- Blaise F., Remy E., Meyer M., Zhou L., Narcy J.P., Roux J. A critical assessment of Agrobacterium tumefaciens‐mediated transformation as a tool for pathogenicity gene discovery in the phytopathogenic fungus Leptosphaeria maculans. Fungal Genet Biol. 2007;44:123–138. et al. - PubMed
-
- Bundock P., Van Atticum H., Dulk‐Ras A., Hooykaas P.J. Insertional mutagenesis in yeast using T‐DNA from Agrobacterium tumefaciens. Yeast. 2002;19:529–536. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

