Constructing and testing the thermodynamic limits of synthetic NAD(P)H:H2 pathways
- PMID: 21261858
- PMCID: PMC3815245
- DOI: 10.1111/j.1751-7915.2008.00033.x
Constructing and testing the thermodynamic limits of synthetic NAD(P)H:H2 pathways
Abstract
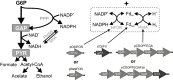
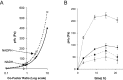
NAD(P)H:H(2) pathways are theoretically predicted to reach equilibrium at very low partial headspace H(2) pressure. An evaluation of the directionality of such near-equilibrium pathways in vivo, using a defined experimental system, is therefore important in order to determine its potential for application. Many anaerobic microorganisms have evolved NAD(P)H:H(2) pathways; however, they are either not genetically tractable, and/or contain multiple H(2) synthesis/consumption pathways linked with other more thermodynamically favourable substrates, such as pyruvate. We therefore constructed a synthetic ferredoxin-dependent NAD(P)H:H(2) pathway model system in Escherichia coli BL21(DE3) and experimentally evaluated the thermodynamic limitations of nucleotide pyridine-dependent H(2) synthesis under closed batch conditions. NADPH-dependent H(2) accumulation was observed with a maximum partial H(2) pressure equivalent to a biochemically effective intracellular NADPH/NADP(+) ratio of 13:1. The molar yield of the NADPH:H(2) pathway was restricted by thermodynamic limitations as it was strongly dependent on the headspace:liquid ratio of the culture vessels. When the substrate specificity was extended to NADH, only the reverse pathway directionality, H(2) consumption, was observed above a partial H(2) pressure of 40 Pa. Substitution of NADH with NADPH or other intermediates, as the main electron acceptor/donor of glucose catabolism and precursor of H(2), is more likely to be applicable for H(2) production.
© 2008 The Authors. Journal compilation © 2008 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures


Similar articles
-
Construction of a synthetic YdbK-dependent pyruvate:H2 pathway in Escherichia coli BL21(DE3).Metab Eng. 2009 May;11(3):139-47. doi: 10.1016/j.ymben.2009.01.002. Epub 2009 Jan 25. Metab Eng. 2009. PMID: 19558967
-
Kinetic, spectroscopic and thermodynamic characterization of the Mycobacterium tuberculosis adrenodoxin reductase homologue FprA.Biochem J. 2003 Jun 1;372(Pt 2):317-27. doi: 10.1042/BJ20021692. Biochem J. 2003. PMID: 12614197 Free PMC article.
-
The effect of NAPRTase overexpression on the total levels of NAD, the NADH/NAD+ ratio, and the distribution of metabolites in Escherichia coli.Metab Eng. 2002 Jul;4(3):238-47. doi: 10.1006/mben.2002.0229. Metab Eng. 2002. PMID: 12616693
-
New approaches to NAD(P)H regeneration in the biosynthesis systems.World J Microbiol Biotechnol. 2018 Sep 10;34(10):141. doi: 10.1007/s11274-018-2530-8. World J Microbiol Biotechnol. 2018. PMID: 30203299 Review.
-
Pyridine nucleotide regulation of cardiac intermediary metabolism.Circ Res. 2012 Aug 17;111(5):628-41. doi: 10.1161/CIRCRESAHA.111.246371. Circ Res. 2012. PMID: 22904042 Review.
Cited by
-
Physiological characteristics of the extreme thermophile Caldicellulosiruptor saccharolyticus: an efficient hydrogen cell factory.Microb Cell Fact. 2010 Nov 22;9:89. doi: 10.1186/1475-2859-9-89. Microb Cell Fact. 2010. PMID: 21092203 Free PMC article. Review.
-
Hydrogen production by recombinant Escherichia coli strains.Microb Biotechnol. 2012 Mar;5(2):214-25. doi: 10.1111/j.1751-7915.2011.00282.x. Epub 2011 Sep 6. Microb Biotechnol. 2012. PMID: 21895995 Free PMC article. Review.
-
Limits in energy generation and biotechnology of primary and secondary products.Microb Biotechnol. 2008 Sep;1(5):343-4. doi: 10.1111/j.1751-7915.2008.00052.x. Microb Biotechnol. 2008. PMID: 21261854 Free PMC article. No abstract available.
-
Enabling technology and core theory of synthetic biology.Sci China Life Sci. 2023 Aug;66(8):1742-1785. doi: 10.1007/s11427-022-2214-2. Epub 2023 Feb 6. Sci China Life Sci. 2023. PMID: 36753021 Free PMC article. Review.
-
An in vitro synthetic biology platform for emerging industrial biomanufacturing: Bottom-up pathway design.Synth Syst Biotechnol. 2018 May 30;3(3):186-195. doi: 10.1016/j.synbio.2018.05.002. eCollection 2018 Sep. Synth Syst Biotechnol. 2018. PMID: 30345404 Free PMC article. Review.
References
-
- Akhtar M.K., Jones P.R. Deletion of iscR stimulates recombinant clostridial Fe–Fe hydrogenase activity and H2‐accumulation in Escherichia coli BL21(DE3) Appl Microbiol Biotechnol. 2008a;78:853–862. - PubMed
-
- Akhtar M.K., Jones P.R. Engineering of a synthetic hydF‐hydE‐hydG‐hydA. Anal Biochem. 2008b;373:170–172. , and operon for biohydrogen production. - PubMed
-
- Alberty R.A. Standard apparent reduction potentials for biochemical half reactions as a function of pH and ionic strength. Arch Biochem Biophys. 2001;389:94–109. - PubMed
-
- Andersen K.B., Von Meyenburg K. Charges of nicotinamide adenine nucleotides and adenylate energy charge as regulatory parameters of the metabolism in Escherichia coli. J Biol Chem. 1977;252:4151–4156. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

