Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain
- PMID: 21261870
- PMCID: PMC3815291
- DOI: 10.1111/j.1751-7915.2008.00050.x
Identification of furfural as a key toxin in lignocellulosic hydrolysates and evolution of a tolerant yeast strain
Abstract
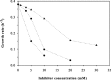
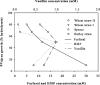
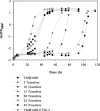
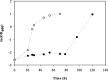
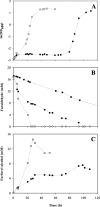
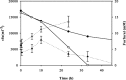
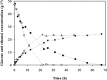
The production of fuel ethanol from low-cost lignocellulosic biomass currently suffers from several limitations. One of them is the presence of inhibitors in lignocellulosic hydrolysates that are released during pre-treatment. These compounds inhibit growth and hamper the production of ethanol, thereby affecting process economics. To delineate the effects of such complex mixtures, we conducted a chemical analysis of four different real-world lignocellulosic hydrolysates and determined their toxicological effect on yeast. By correlating the potential inhibitor abundance to the growth-inhibiting properties of the corresponding hydrolysates, we identified furfural as an important contributor to hydrolysate toxicity for yeast. Subsequently, we conducted a targeted evolution experiment to improve growth behaviour of the half industrial Saccharomyces cerevisiae strain TMB3400 in the hydrolysates. After about 300 generations, representative clones from these evolved populations exhibited significantly reduced lag phases in medium containing the single inhibitor furfural, but also in hydrolysate-supplemented medium. Furthermore, these strains were able to grow at concentrations of hydrolysates that effectively killed the parental strain and exhibited significantly improved bioconversion characteristics under industrially relevant conditions. The improved resistance of our evolved strains was based on their capacity to remain viable in a toxic environment during the prolonged, furfural induced lag phase.
© 2008 The Authors. Journal compilation © 2008 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures
References
-
- Alkasrawi M., Rudolf A., Liden G., Zacchi G. Influence of strain and cultivation procedure on the performance of simultaneous saccharification and fermentation of steam pretreated spruce. Enzyme Microb Technol. 2006;38:279–286.
-
- Almeida J.R.M., Modig T., Petersson A., Hahn‐Hagerdal B., Liden G., Gorwa‐Grauslund M.F. Increased tolerance and conversion of inhibitors in lignocellulosic hydrolysates by Saccharomyces cerevisiae. J Chem Technol Biotechnol. 2007;82:340–349.
-
- Delgenes J.P., Moletta R., Navarro J.M. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilisPichia stipitis, and Candida shehatae. Enzyme Microb Technol. 1996;19:220–225.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

