Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this
- PMID: 21261911
- PMCID: PMC3815837
- DOI: 10.1111/j.1751-7915.2008.00078.x
Microbial degradation of lignin: how a bulky recalcitrant polymer is efficiently recycled in nature and how we can take advantage of this
Abstract
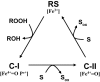
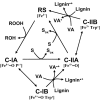
Lignin is the second most abundant constituent of the cell wall of vascular plants, where it protects cellulose towards hydrolytic attack by saprophytic and pathogenic microbes. Its removal represents a key step for carbon recycling in land ecosystems, as well as a central issue for industrial utilization of plant biomass. The lignin polymer is highly recalcitrant towards chemical and biological degradation due to its molecular architecture, where different non-phenolic phenylpropanoid units form a complex three-dimensional network linked by a variety of ether and carbon-carbon bonds. Ligninolytic microbes have developed a unique strategy to handle lignin degradation based on unspecific one-electron oxidation of the benzenic rings in the different lignin substructures by extracellular haemperoxidases acting synergistically with peroxide-generating oxidases. These peroxidases poses two outstanding characteristics: (i) they have unusually high redox potential due to haem pocket architecture that enables oxidation of non-phenolic aromatic rings, and (ii) they are able to generate a protein oxidizer by electron transfer to the haem cofactor forming a catalytic tryptophanyl-free radical at the protein surface, where it can interact with the bulky lignin polymer. The structure-function information currently available is being used to build tailor-made peroxidases and other oxidoreductases as industrial biocatalysts.
© 2009 The Authors. Journal compilation © 2009 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures



References
-
- Adler E. Lignin chemistry – past, present and future. Wood Sci Technol. 1977;11:169–218.
-
- Bajpai P. Biological bleaching of chemical pulps. Crit Rev Biotechnol. 2004;24:1–58. - PubMed
-
- Baldrian P. Fungal laccases – occurrence and properties. FEMS Microbiol Rev. 2006;30:215–242. - PubMed
-
- Banci L. Structural properties of peroxidases. J Biotechnol. 1997;53:253–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

