A molecular key for building hyphae aggregates: the role of the newly identified Streptomyces protein HyaS
- PMID: 21261929
- PMCID: PMC3815755
- DOI: 10.1111/j.1751-7915.2009.00093.x
A molecular key for building hyphae aggregates: the role of the newly identified Streptomyces protein HyaS
Abstract
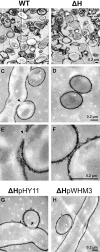
Streptomycetes produce many metabolites with medical and biotechnological applications. During fermentations, their hyphae build aggregates, a process in which the newly identified protein HyaS plays an important role. The corresponding hyaS gene is present within all investigated Streptomyces species. Reporter fusions indicate that transcription of hyaS occurs within substrate hyphae of the Streptomyces lividans wild type (WT). The HyaS protein is dominantly associated with the substrate hyphae. The WT strain forms cylindrically shaped clumps of densely packed substrate hyphae, often fusing to higher aggregates (pellets), which remain stably associated during shaking. Investigations by electron microscopy suggest that HyaS induces tight fusion-like contacts among substrate hyphae. In contrast, the pellets of the designed hyaS disruption mutant ΔH are irregular in shape, contain frequently outgrowing bunches of hyphae, and fuse less frequently. ΔH complemented with a plasmid carrying hyaS resembles the WT phenotype. Biochemical studies indicate that the C-terminal region of HyaS has amine oxidase activity. Investigations of ΔH transformants, each carrying a specifically mutated gene, lead to the conclusion that the in situ oxidase activity correlates with the pellet-inducing role of HyaS, and depends on the presence of certain histidine residues. Furthermore, the level of undecylprodigiosin, a red pigment with antibiotic activity, is influenced by the engineered hyaS subtype within a strain. These data present the first molecular basis for future manipulation of pellets, and concomitant production of secondary metabolites during biotechnological processes.
© 2009 The Authors. Journal compilation © 2009 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures







References
-
- Baltz R.H. Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat Biotechnol. 2006;24:1533–1540. - PubMed
-
- Bentley S.D., Chater K.F., Cerdeno‐Tarraga A.M., Challis G.L., Thomson N.R., James K.D. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2) Nature. 2002;417:141–147. et al. - PubMed
-
- Blake M.S., Johnston K.H., Russel‐Jones G.J., Gotschlich E.C. A rapid, sensitive method for detection of alkaline phosphatase‐conjugated anti‐antibody on Western‐blots. Anal Biochem. 1984;136:175–179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

