GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice
- PMID: 21262227
- PMCID: PMC3541694
- DOI: 10.1053/j.gastro.2011.01.033
GATA factors regulate proliferation, differentiation, and gene expression in small intestine of mature mice
Abstract
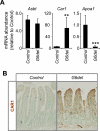
Background & aims: GATA transcription factors regulate proliferation, differentiation, and gene expression in multiple organs. GATA4 is expressed in the proximal 85% of the small intestine and regulates the jejunal-ileal gradient in absorptive enterocyte gene expression. GATA6 is co-expressed with GATA4 but also is expressed in the ileum; its function in the mature small intestine is unknown.
Methods: We investigated the function of GATA6 in small intestine using adult mice with conditional, inducible deletion of Gata6, or Gata6 and Gata4, specifically in the intestine.
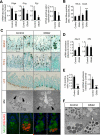
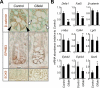
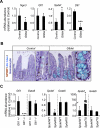
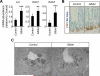
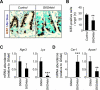
Results: In ileum, deletion of Gata6 caused a decrease in crypt cell proliferation and numbers of enteroendocrine and Paneth cells, an increase in numbers of goblet-like cells in crypts, and altered expression of genes specific to absorptive enterocytes. In contrast to ileum, deletion of Gata6 caused an increase in numbers of Paneth cells in jejunum and ileum. Deletion of Gata6 and Gata4 resulted in a jejunal and duodenal phenotype that was nearly identical to that in the ileum after deletion of Gata6 alone, revealing common functions for GATA6 and GATA4.
Conclusions: GATA transcription factors are required for crypt cell proliferation, secretory cell differentiation, and absorptive enterocyte gene expression in the small intestinal epithelium.
Copyright © 2011 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Stappenbeck TS, Wong MH, Saam JR, et al. Notes from some crypt watchers: regulation of renewal in the mouse intestinal epithelium. Curr Opin Cell Biol. 1998;10:702–9. - PubMed
-
- Ireland H, Kemp R, Houghton C, et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–46. - PubMed
-
- Korinek V, Barker N, Moerer P, et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. Nat Genet. 1998;19:379–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK061382/DK/NIDDK NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- R01 DK066226/DK/NIDDK NIH HHS/United States
- R01-CA-142826/CA/NCI NIH HHS/United States
- R03-DK-84167/DK/NIDDK NIH HHS/United States
- R01-DK-054111/DK/NIDDK NIH HHS/United States
- R01-DK-061382/DK/NIDDK NIH HHS/United States
- 5P30-DK-34854/DK/NIDDK NIH HHS/United States
- R01-DK-066226/DK/NIDDK NIH HHS/United States
- R01 DK055743/DK/NIDDK NIH HHS/United States
- R01 DK054111/DK/NIDDK NIH HHS/United States
- R01 CA142826/CA/NCI NIH HHS/United States
- R01-DK-055743/DK/NIDDK NIH HHS/United States
- R03 DK084167/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

