The molecular and cellular basis of bitter taste in Drosophila
- PMID: 21262465
- PMCID: PMC3033050
- DOI: 10.1016/j.neuron.2011.01.001
The molecular and cellular basis of bitter taste in Drosophila
Abstract
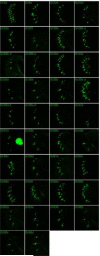
The extent of diversity among bitter-sensing neurons is a fundamental issue in the field of taste. Data are limited and conflicting as to whether bitter neurons are broadly tuned and uniform, resulting in indiscriminate avoidance of bitter stimuli, or diverse, allowing a more discerning evaluation of food sources. We provide a systematic analysis of how bitter taste is encoded by the major taste organ of the Drosophila head, the labellum. Each of 16 bitter compounds is tested physiologically against all 31 taste hairs, revealing responses that are diverse in magnitude and dynamics. Four functional classes of bitter neurons are defined. Four corresponding classes are defined through expression analysis of all 68 gustatory taste receptors. A receptor-to-neuron-to-tastant map is constructed. Misexpression of one receptor confers bitter responses as predicted by the map. These results reveal a degree of complexity that greatly expands the capacity of the system to encode bitter taste.
© 2011 Elsevier Inc. All rights reserved.
Figures








References
-
- Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

