Using the structure of inhibitory networks to unravel mechanisms of spatiotemporal patterning
- PMID: 21262473
- PMCID: PMC3150555
- DOI: 10.1016/j.neuron.2010.12.019
Using the structure of inhibitory networks to unravel mechanisms of spatiotemporal patterning
Abstract
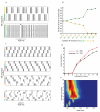
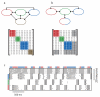
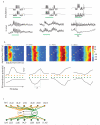
Neuronal networks exhibit a rich dynamical repertoire, a consequence of both the intrinsic properties of neurons and the structure of the network. It has been hypothesized that inhibitory interneurons corral principal neurons into transiently synchronous ensembles that encode sensory information and subserve behavior. How does the structure of the inhibitory network facilitate such spatiotemporal patterning? We established a relationship between an important structural property of a network, its colorings, and the dynamics it constrains. Using a model of the insect antennal lobe, we show that our description allows the explicit identification of the groups of inhibitory interneurons that switch, during odor stimulation, between activity and quiescence in a coordinated manner determined by features of the network structure. This description optimally matches the perspective of the downstream neurons looking for synchrony in ensembles of presynaptic cells and allows a low-dimensional description of seemingly complex high-dimensional network activity.
© 2011 Elsevier Inc. All rights reserved.
Figures




References
-
- Abeles M, Bergman H, Margalit E, Vaadia E. Spatiotemporal firing patterns in the frontal cortex of behaving monkeys. J Neurophysiol. 1993;70:1629–1638. - PubMed
-
- Appel K, Haken W. Every planar map is four colorable. Illinois Journal of Mathematics. 1977a;21:439–567.
-
- Appel K, Haken W. Solution of the four color map problem. Scientific American. 1977b;237:108–121.
-
- Arenas A, Diaz-Guilera A, Kurths J, Moreno Y, Zhou C. Synchronization in complex networks. Physics Reports. 2008;469:93–153.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

