Functional expression of transgenic 1sDHPR channels in adult mammalian skeletal muscle fibres
- PMID: 21262876
- PMCID: PMC3082101
- DOI: 10.1113/jphysiol.2010.202804
Functional expression of transgenic 1sDHPR channels in adult mammalian skeletal muscle fibres
Abstract
We investigated the effects of the overexpression of two enhanced green fluorescent protein (EGFP)-tagged α1sDHPR variants on Ca2+ currents (ICa), charge movements (Q) and SR Ca2+ release of muscle fibres isolated from adult mice. Flexor digitorum brevis (FDB)muscles were transfected by in vivo electroporation with plasmids encoding for EGFP-α1sDHPR-wt and EGFP-α1sDHPR-T935Y (an isradipine-insensitive mutant). Two-photon laser scanning microscopy (TPLSM) was used to study the subcellular localization of transgenic proteins, while ICa, Q and Ca2+ release were studied electrophysiologically and optically under voltage-clamp conditions. TPLSM images demonstrated that most of the transgenic α1sDHPR was correctly targeted to the transverse tubular system (TTS). Immunoblotting analysis of crude extracts of transfected fibres demonstrated the synthesis of bona fide transgenic EGFP-α1sDHPR-wt in quantities comparable to that of native α1sDHPR. Though expression of both transgenic variants of the alpha subunit of the dihydropyridine receptor (α1sDHPR) resulted in ∼50% increase in Q, they surprisingly had no effect on the maximal Ca2+ conductance (gCa) nor the SR Ca2+ release. Nonetheless, fibres expressing EGFP-α1sDHPR-T935Y exhibited up to 70% isradipine-insensitive ICa (ICa-ins) with a right-shifted voltage dependence compared to that in control fibres. Interestingly, Qand SRCa2+ release also displayed right-shifted voltage dependence in fibres expressing EGFP-α1sDHPR-T935Y. In contrast, the midpoints of the voltage dependence of gCa, Q and Ca2+ release were not different from those in control fibres and in fibres expressing EGFP-α1sDHPR-wt. Overall, our results suggest that transgenic α1sDHPRs are correctly trafficked and inserted in the TTS membrane, and that a substantial fraction of the mworks as conductive Ca2+ channels capable of physiologically controlling the release of Ca2+ from the SR. A plausible corollary of this work is that the expression of transgenic variants of the α1sDHPR leads to the replacement of native channels interacting with the ryanodine receptor 1 (RyR1), thus demonstrating the feasibility of molecular remodelling of the triads in adult skeletal muscle fibres.
Figures

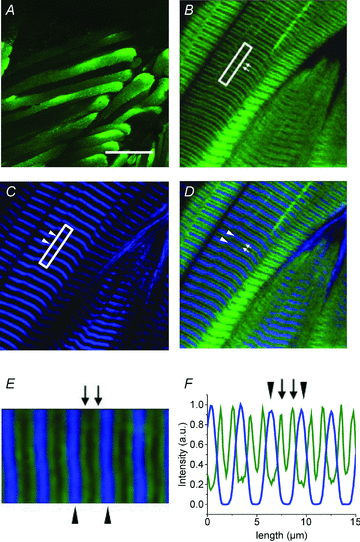

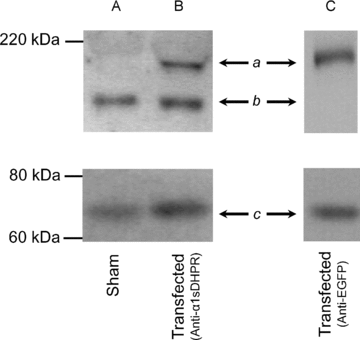

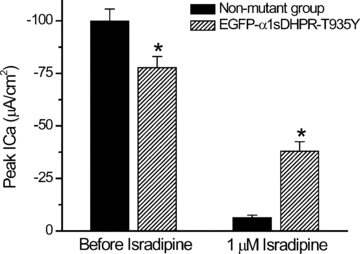


Comment in
-
A shortcut to a skeletal muscle DHPR knock-in?J Physiol. 2011 Oct 1;589(Pt 19):4645-6. doi: 10.1113/jphysiol.2011.216234. J Physiol. 2011. PMID: 21965631 Free PMC article. No abstract available.
References
-
- Adams BA, Beam KG. Muscular dysgenesis in mice: a model system for studying excitation-contraction coupling. FASEB J. 1990;4:2809–2816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

